Abstract
Objective
Because menisci and the M:L BMD are associated with loading within the knee, we postulated there to be an association between compartment-specific meniscal damage and M:L BMD. We hypothesized that knees with higher M:L BMD, consistent with increased medial subchondral BMD, would be associated with medial meniscal damage, and lower ratios with lateral meniscal damage.
Methods
We conducted a cross-sectional study evaluating participants in the Framingham OA Cohort having MRIs, BMDs, and x-rays of the knee. Medial and lateral meniscal damage were defined on MRI. We performed a logistic regression with medial meniscal damage as the outcome testing M:L BMD groups as predictor variables. We adjusted for age and sex; we used GEE to adjust for correlation between knees. Identical analyses were performed evaluating lateral meniscal damage.
Results
When evaluating the relation of M:L BMD to medial meniscal damage, the odds ratios (ORs) of prevalent medial meniscal damage from lowest to highest quartile of M:L BMD were 1.0 (referent), 1.9, 2.4 and 8.9, p for trend <0.0001. When evaluating the relation of M:L BMD to lateral meniscal damage, the ORs of prevalent lateral meniscal damage from lowest to highest quartile of M:L BMD were 1.0 (referent), 0.3, 0.2, and 0.2, p for trend =0.001.
Conclusions
Meniscal damage is associated with higher regional tibial BMD in the same compartment. Our findings highlight the close relationship between meniscal integrity and regional tibial subchondral BMD.
Introduction
Menisci are fibrocartilaginous structures located between the tibia and the femur, attached to the superior aspect of the tibial plateau. These structures play a large role in load distribution in the knee.1 A knee without an intact meniscus experiences twice the peak pressures upon loading compared to a knee with an intact meniscus in place.2 Knees with meniscectomies, even partial or limited, are at greatly increased risk for the development of OA.3–6 However, the role meniscal damage plays in OA pathophysiology is unclear. It is known that meniscal damage, including meniscal tears, are highly prevalent in OA,7 and recently it has been shown that these meniscal changes are predictive of structural progression of OA longitudinally.8
The medial versus lateral tibial plateau bone mineral density ratio (M:L BMD) is a measure of relative bone mineral density in the medial as compared to the lateral tibial plateau. Because the region of interest averages the bone within the region, it is unable to provide detail on the specifics of trabecular mineralization or subchondral plate thickness. That being said, DXA technology is widely available and the ascertainment of BMDs is automated which makes this measurement of bone appealing. Furthermore, it is a measure that has been associated with established features of OA9–11 that focuses on the subchondral bone of the tibial plateau. A higher ratio is consistent with the medial tibial BMD being greater than the lateral BMD; similarly, a lower ratio is consistent with the lateral tibial BMD being higher than the medial BMD. The M:L BMD has also been associated11–13 with established biomechanical risk factors for OA progression including static alignment14 and dynamic alignment,15, 16 both measures of regional loading within a knee. There is also evidence to suggest that unloading a knee is associated with a normalization of the M:L BMD.17, 18
Because menisci and the M:L BMD are associated with loading within the knee, we postulated there to be an association between compartment-specific meniscal damage and M:L BMD, so that an increase in M:L BMD would be associated with medial meniscal damage, and likewise, a lower M:L BMD would be associated with lateral damage. The purpose of this study was to explore the cross-sectional relationship between meniscal damage and M:L BMD in a population based study, the Framingham OA Cohort.
Materials and Methods
Subjects
Our population-based study cohort consisted of two separate groups, members of 1) the Framingham Heart Study Offspring Cohort and 2) a newly recruited cohort from the town of Framingham, Massachusetts. Participants of this combined group, designated the Framingham OA Study Cohort, were examined between 2002 – 2005.
The Framingham Heart Study Offspring Cohort participants included surviving descendants (and spouses of descendants) of the Original Framingham Heart Study cohort subjects.19 As part of a study of the inheritance of OA, selected participants were originally examined in 1992–94. Members of this group were identified as potential participants of the current study. All were contacted by telephone and invited to participate in the study. Using a validated survey instrument20 supplemented by questions about medication use that would reflect treated rheumatoid arthritis, people who screened positively for rheumatoid arthritis were excluded.
The newly recruited cohort participants were drawn from a random sample of the Framingham, Massachusetts community. Flyers were hung in public areas to increase awareness of the study which was focused on health including bone health, foot health and arthritis. Participants were recruited using random digit dialing and census tract data to ensure a representative sample of the Framingham community. To be included, subjects had to be at least 50 years old and ambulatory (use of assistive devices such as canes and walkers was allowed). Exclusion criteria were the presence of bilateral total knee replacements and a positive screen for rheumatoid arthritis as above.20 In neither group was participant selection based on the presence or absence of knee OA.
All of those who agreed to participate in the Framingham OA Study Cohort had tibial plateau BMD assessments and plain PA radiographs of both knees, except for those knees status-post total joint replacement. There was a 2 month period where, due to time constraints, the tibial plateau BMD assessments were only obtained on the right knee.
Persons with no contraindications had an MRI performed of at least one knee. Among the Framingham Offspring Cohort, all participants with pain in at least one knee had MRIs performed of both knees. The question used to assess for knee pain was “In the past 30 days, have you had any pain, aching or stiffness in either of your knees?” All members of the newly recruited cohort had an MRI performed of one knee only. Those with a right total knee replacement had a left knee MRI. All others had a right knee MRI.
Age, sex, and body mass index (BMI) were assessed in all study participants. Body mass index (BMI) was calculated using the Quetelet’s index (weight/height2) as kg/m2.21 Height was measured to the nearest 0.25 inch using a stadiometer and weight to the nearest 0.25 pound using a balance beam scale with shoes and heavy clothing removed.
Protocol for obtaining Magnetic Resonance Imaging (MRI)
All studies were performed with a Siemens 1.5T MRI system (Mountain View, CA) using a phased-array knee coil. A positioning device was used to ensure uniform placement of the knee among patients. T2-weighted fat-suppressed images in axial, sagittal and coronal planes were acquired with the following pulse sequence parameters: TR (recovery time) = 3610 msec, TE (lapse time) = 40 msec, slice thickness of 3.5 mm, and field of view of 14 cm. T1-weighted spin echo images in the sagittal plane were acquired, using the following pulse sequence parameters: TR = 475 msec, TE = 24 msec, slice thickness 3.5 mm, and field of view of 14 cm.
Scoring of MRI Images
Each MRI was scored by one reader; in total 2 MRI readers were utilized in this study. Meniscal damage was scored on MRI in the anterior horn, the body, and the posterior horn of each medial and lateral meniscus using the Whole Organ MRI Scoring (WORMS) system where a score of 0 = intact meniscus, 1 = minor radial tear or parrot-beak tear, 2 = nondisplaced tear or prior surgical repair, 3 = displaced or partial resection or maceration, and 4 = complete maceration.22 The inter-observer weighted kappa for these measures was 0.66. These meniscal measurements were used to assess for presence/absence of damage in the medial and lateral menisci. Medial meniscal damage was dichotomously defined as WORMS score ≥ 1 in the medial meniscal anterior horn, the body and/or the posterior horn, and lateral meniscal damage was similarly defined. The cut point of ≥ 1 was used as this allowed for discrimination of menisci that had any damage as compared to those with no damage. Based on these definitions, each knee was given a meniscal damage status of one of the following: 1) No meniscal damage, 2) Medial meniscal damage only, 3) Lateral meniscal damage only, or 4) Medial and lateral meniscal damage.
Plain radiographs of the knee
A fixed flexion PA view of both knees was obtained with weight-bearing as described by Carbone et al.23 These films were scored for Kellgren and Lawrence (K/L) grade (0 – 4)24 (weighted kappa [intra-rater reliability] = 0.83 (95% CI: 0.74 – 0.91)) by an academically based bone and joint radiologist blinded to the M:L BMD Ratios and to the MRI images. Those knees with a K/L score ≥ 2 were defined as having radiographic OA.
Protocol for obtaining tibial plateau BMD measurements
The proximal tibiae were scanned using dual x-ray absorptiometry (DXA) (Lunar Prodigy scanner GE Lunar Corp., Madison WI, USA), using the spine analysis option. The lower extremity was positioned with the direction of scanning perpendicular to the long axis of the tibia, and neutrally rotated. Also, one 5 pound rice bag was positioned posterior to the popliteal fossa to place the knee in mild flexion. As the scanner requires the presence of surrounding soft tissue to set the threshold for bony tissue, and since little soft tissue exists around the knee joint, we mimicked “soft tissue” surrounding the knee by placing multiple additional 5 pound rice bags (usually between 6 and 7 bags) circumferentially around the knee, making sure there were no pockets of air between the knee and the bags. Stacking the rice bags also provided stabilization, minimizing motion of the knee of interest. The positioning laser light was used to position the center of the scanner arm 2 inches below the inferior pole of the patella.
We created 2 customized regions of interest (ROIs) for each knee, according to a protocol outlined by Hurwitz et al.13 The scan included the proximal 20 mm of the tibia. The width of each ROI (i.e. medial and lateral ROIs), in the medio-lateral direction was set as ½ the distance between the medial and lateral bone edges along a line midway between the far medial and lateral points of the tibial plateau. The 2 regions were positioned so that their top edges were just superior and parallel to the medial and lateral joint surfaces of the tibia. For each ROI, the BMD was measured in the area bounded by the bone edges and the boundaries of the region positioned within the bone. These ROI locations were chosen to evaluate the BMD of the lateral and medial areas of the subchondral cancellous bone and the cortical bone of the diaphysis. The 20 mm height of the ROIs was chosen so that the fibula was largely excluded from the measurements.
M:L BMD were calculated by dividing the medial tibial plateau BMD (g/cm2) by the lateral tibial plateau BMD (g/cm2). The test-retest (with repositioning) intraclass correlation was 0.99 for both the medial BMD and lateral BMD, and 0.96 for the M:L BMD.9 Excess medial loading and excess lateral loading were defined as those knees in the highest and lowest quartiles of M:L BMD respectively.
Statistical analysis
All knees that had MRIs, BMDs, and plain radiographs, in addition to participant data on age, sex, and BMI were included in the following analyses.
We described demographic characteristics including age, sex, body mass index (BMI) and the prevalence of radiographic OA in the whole cohort and then in those with no meniscal damage, medial meniscal damage only, lateral meniscal damage only, and with both medial and lateral meniscal damage.
We assessed for the correlation among maximal WORMS meniscal score in the medial and lateral menisci and the M:L BMD, restricted to right knees.
We graphically described the distribution of M:L BMD partitioned by meniscal damage status using four groups: 1) No meniscal damage, 2) Medial meniscal damage only, 3) Lateral meniscal damage only 4) Both medial and lateral meniscal damage. We used an analysis of variance (ANOVA) including only the right knee to evaluate whether there were differences in mean M:L BMD among the 4 meniscal damage groups using a 2 sided alpha of 0.05, controlling for age, sex, and BMI. When making individual comparisons among the 4 meniscal damage groups, p-values were adjusted for multiple comparisons using the Scheffe procedure.25 We repeated these analyses stratifying by the presence/absence of radiographic OA.
We then performed a logistic regression including all knees, with presence/absence of medial meniscal damage as the dependent variable and case-based quartiles of M:L BMD as the predictor variables. The analysis was adjusted for age and sex; generalized estimating equations (GEE) were used to adjust for correlation between two knees within an individual.26 Identical analyses were performed evaluating lateral meniscal damage as the outcome. In order to test the effect of utilizing different cut points for the definition of meniscal damage, the above analyses were repeated utilizing definitions of Medial meniscal damage was dichotomously defined as WORMS score ≥ 2 and 3 in the medial meniscal anterior horn, the body and/or the posterior horn, and lateral meniscal damage was similarly defined.
Statistical analyses were performed using the SAS system for Windows (version 9.1; SAS Institute, Cary, NC). P-values <0.05 were considered statistically significant.
Results
845 participants (913 knees) were included in this study, 58% were female, with a mean age of 63.6 (SD 8.8), mean BMI of 28.5 (SD 5.5). 170/913 knees (19%) had radiographic evidence of OA. 309/913 (34%) had at least one meniscus that was damaged, 255/913 (28%) had medial meniscal damage, 101/913 (11%) had lateral meniscal damage, and 47/913 (5%) had damage of both menisci.
There were differences in participants based on the meniscal damage status of their knees as seen in Table 1. Those with no meniscal damage were the youngest; those with medial meniscal damage only, lateral meniscal damage only, and both medial and lateral meniscal damage were respectively compared with the prior group with mean ages of 62.0, 65.5, 67.4, and 72.2. Also, those with medial meniscal damage had a higher proportion of males and had a higher BMI as compared to those without medial meniscal damage. Some knees with no meniscal damage did have evidence of radiographic OA (7%), while those with one meniscus damaged had higher prevalences of radiographic OA (medial meniscus 39% and lateral meniscus 31%). Those with both medial and lateral menisci damage had an even higher prevalence of radiographic OA (60%).
Table 1.
Characteristics of knees based on meniscal status (n=914 knees)
| No Meniscal Damage n = 604 | Medial Meniscal Damage Only n = 208 | Lateral Meniscal Damage Only n = 54 | Medial and Lateral Meniscal Damage n = 47 | |
|---|---|---|---|---|
| Age (years) (Mean (SD)) | 62.0 (8.3) | 65.5 (8.7) | 67.4 (8.5) | 72.2 (8.2) |
| Sex | Female 62% | Female 49% | Female 63% | Female 49% |
| Male 38% | Male 51% | Male 37% | Male 51% | |
| BMI (kg/m2) (Mean (SD)) | 28.2 (5.4) | 29.1 (5.8) | 28.0 (5.1) | 29.4 (6.0) |
| Kellgren/Lawrence ≥ 2 | 43/605 (7%) | 82/208 (39%) | 17/54 (31%) | 28/47 (60%) |
The prevalence of medial meniscal damage among those with radiographic OA (110/170 (64.7%)) was higher compared with those without radiographic OA (145/743 (19.5%)). Similarly the prevalence of lateral meniscal damage among those with radiographic OA 45/170 (26.5%) was higher than those without radiographic OA (56/743 (7.5%)).
There was a moderate positive correlation of M:L BMD with maximal medial meniscal score, R = 0.39 (p<0.0001), so that a higher ratio was associated with a larger maximal medial meniscal damage score (Table 2). There was also a significant negative correlation of M:L BMD with maximal lateral meniscal score, R = −0.19 (p<0.0001) so that a lower ratio was associated with a higher maximal lateral meniscal score. We also observed a significant positive correlation between maximum medial meniscal score and maximum lateral meniscal score, R = 0.15 (p<0.0001).
Table 2.
Correlations of M:L BMD, medial meniscal score and lateral meniscal scores, restricted to right knees. (N=820)
| M:L BMD | Maximum Medial Meniscal Score | Maximum Lateral Meniscal Score | |
|---|---|---|---|
| M:L BMD | 1.0000 | 0.39 p<0.0001 | − 0.19 p<0.0001 |
| Maximum Medial Meniscal Score | 1.0000 | 0.15 p<0.0001 | |
| Maximum Lateral Meniscal Score | 1.0000 |
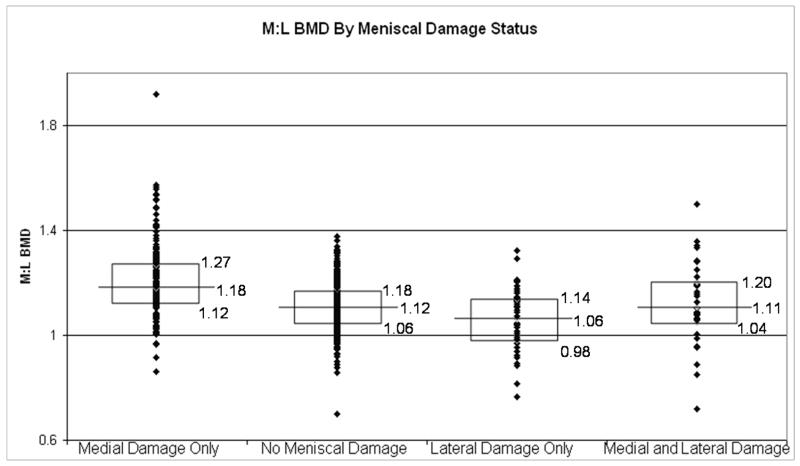
In Figure 1, representing all right knees in this cohort, those with medial meniscal damage had higher M:L BMDs, as compared with those with no meniscal tears (p < 0.0001). Likewise those knees with lateral meniscal damage had lower M:L BMDs, compared with knees with no meniscal tears (p=0.0026). The knees with both medial and lateral meniscal damage had M:L BMD that were similar to those with no meniscal tears (p = .96). These findings persisted, though the magnitude of the differences was smaller among those without radiographic OA (Figure 2), and was more pronounced among those with radiographic OA (Figure 3).
Figure 1.
Box plots depicting the medians and interquartile ranges of M:L BMDs by meniscal damage status. The ANOVA showed that the differences in M:L BMD among the groups were significant (p<0.0001). Individual comparisons: medial damage only v. no meniscal damage, p < 0.0001; lateral damage only v. no meniscal damage, p = 0.0026; medial and lateral damage v. no meniscal damage, p = 0.96).
Figure 2.
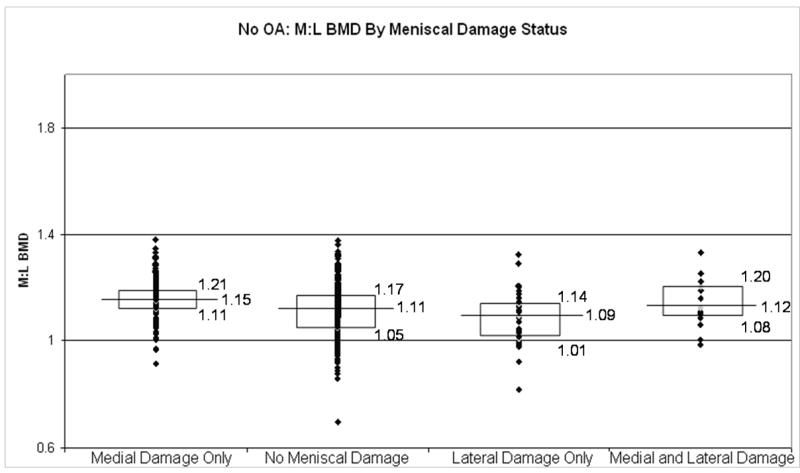
Box plots depicting the medians and interquartile ranges of M:L BMDs by meniscal damage status in those without radiographic OA. Individual comparisons: medial damage only v. no meniscal damage, p = 0.0005; lateral damage only v. no meniscal damage, p = 0.28; medial and lateral damage v. no meniscal damage, p = 0.90).
Figure 3.
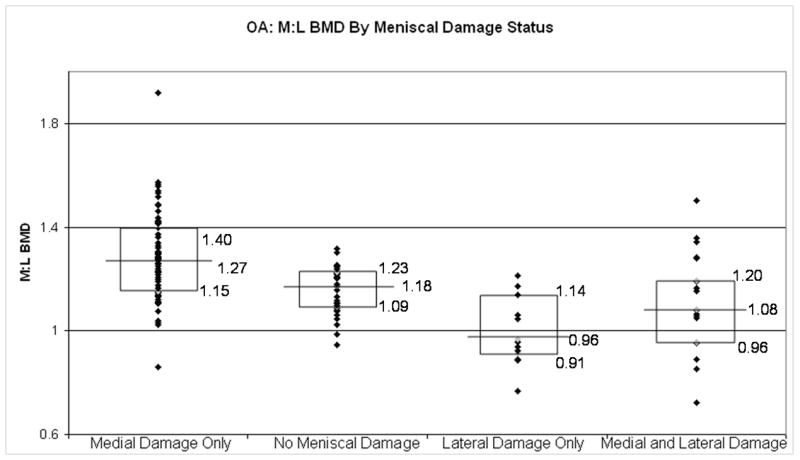
Box plots depicting the medians and interquartile ranges of M:L BMDs by meniscal damage status in those with radiographic OA. Individual comparisons: medial damage only v. no meniscal damage, p = 0.0041; lateral damage only v. no meniscal damage, p = 0.01; medial and lateral damage v. no meniscal damage, p = 0.46).
When evaluating the relation of M:L BMD to medial meniscal damage, the odds ratios (ORs) of prevalent medial meniscal damage from lowest to highest quartile of M:L BMD were 1.0 (referent), 1.9, 2.4 and 8.9, p for trend <0.0001. (Table 3) The 95% CIs for the ORs are presented in Table 3 in parenthesis under the point estimates. Findings were similar among those with and without radiographic evidence of OA. In those with radiographic OA, 52 of the 110 knees with medial meniscal damage (47%) were in the highest M:L BMD quartile such that almost 90% of the knees in that highest quartile had evidence of medial meniscal damage. The results were similar when using medial meniscal damage definitions of maximal meniscal WORMS scores of ≥ 2 and ≥ 3. (Data not shown)
Table 3.
Relation of M:L BMD Ratio Groups to the Prevalence of BMLs
| Medial Meniscal Damage | M:L BMD Ratio Groups
|
||||
|---|---|---|---|---|---|
| 1 (0.70 – 1.11) | 2 (1.11 – 1.18) | 3 (1.18 – 1.27) | 4 (1.27 – 1.91) | p for trend | |
| Adjusted Odds Ratio of Medial Meniscal Damage (95% CIs) | 1.0 (reference) | 1.9 (1.3 – 2.9) | 2.4 (1.6 – 3.6) | 8.9 (5.1 – 15.5) | p<0.0001 |
| Medial Meniscal Damage Prevalence (All knees) | (64/388) 16.5% | (64/233) 27.5% | (63/196) 32.1% | (64/96) 66.7% | ----- |
| Medial Meniscal Damage Prevalence (K/L ≥ 2) | (23/52) 44.2% | (17/26) 65.4% | (18/34) 52.9% | (52/58) 89.7% | ----- |
| Medial Meniscal Damage Prevalence (K/L < 2) | (41/336) 12.2% | (47/207) 22.7% | (45/162) 27.8% | (12/38) 31.6% | ----- |
| Lateral Meniscal Damage | M:L BMD Ratio Groups
|
||||
| 1 (0.70 – 1.00) | 2 (1.00 – 1.09) | 3 (1.09 – 1.19) | 4 (1.19 – 1.91) | p for trend | |
|
| |||||
| Adjusted Odds Ratio of Lateral Meniscal Damage (95% CIs) | 1.0 (reference) | 0.3 (0.1 – 0.5) | 0.2 (0.1 – 0.3) | 0.2 (0.1 – 0.4) | p=0.001 |
| Lateral Meniscal Damage Prevalence (All knees) | (26/87) 29.9% | (24/223) 10.8% | (27/358) 7.5% | (24/245) 9.8% | ----- |
| Lateral Meniscal Damage Prevalence (K/L ≥ 2) | (16/20) 80.0% | (10/25) 40.0% | (7/36) 19.4% | (12/89) 13.5% | ----- |
| Lateral Meniscal Damage Prevalence (K/L < 2) | (10/67) 14.9% | (14/198) 7.1% | (20/322) 6.2% | (12/156) 7.7% | ----- |
Odds Ratios are adjusted for age and sex.
Italicized results are meniscal damage prevalence measurements stratified by radiographic OA status
When evaluating the relation of M:L BMD to lateral meniscal damage, the ORs of prevalent lateral meniscal damage from lowest to highest quartile of M:L BMD were 1.0 (referent), 0.3, 0.2, and 0.2, p for trend =0.001. (Table 3) The 95% CIs for the ORs are presented in Table 3 in parenthesis under the point estimates. Findings were similar among those with and without radiographic evidence of OA. In those with radiographic OA, 16 of the 45 knees with lateral meniscal damage (34%) were in the lowest M:L BMD quartile such that 80% of the knees in that lowest M:L BMD quartile had evidence of lateral meniscal damage. The results were similar when using lateral meniscal damage definitions of maximal meniscal WORMS scores of ≥ 2 and ≥ 3. (Data not shown)
Discussion
This is the first study of humans to show that meniscal damage is associated with regional tibial BMD, a measure of subchondral bone. In this cross-sectional study, we found that the compartment where there was meniscal damage, there was a higher relative regional subchondral tibial BMD where medial meniscal damage was strongly positively associated with M:L BMD and lateral meniscal damage was strongly negatively associated with M:L BMD.
This relationship between meniscal damage and relative regional subchondral BMD was observed both in those without radiographic OA, in those with radiographic OA, specifically the prevalence of medial meniscal damage was higher in the high M:L BMD group (consistent with a high medial BMD) as compared to those in the low M:L BMD group in those with radiographic evidence of OA. A similar relationship was also seen in those without radiographic evidence of OA with the exception that the overall prevalence of meniscal damage was lower in this group as compared to the OA group. Similar findings were observed when looking at the prevalence of lateral meniscal damage and those in the lowest M:L BMD group (consistent with a high lateral BMD). These findings suggest that the relationship between meniscal damage and M:L BMD may develop early and likely continues to be important late in the pathophysiology of OA. Further evidence to support this assertion is that, in figure 2, when evaluating those without radiographic OA, those with medial meniscal damage had a higher M:L BMD compared with those without meniscal damage (1.15 v. 1.11, p = 0.0005). Similarly, those with lateral meniscal damage had a lower M:L BMD compared to those without any meniscal damage, though this did not reach statistical significance. (1.09 v. 1.11, p = 0.28). However, when looking at those with radiographic evidence of OA, in figure 3, the difference in M:L BMD between those with medial meniscal damage only and no meniscal damage (1.27 v. 1.18, p = 0.0041) and between those with lateral meniscal damage only and no meniscal damage (0.96 v. 1.18, p = 0.01) were both larger in magnitude and both statistically significant. While the cross-sectional design makes it difficult to make temporal inferences regarding our findings, identification of the presence of a relationship between subchondral bone changes and meniscal damage is meaningful.
In our logistic regression models, we adjusted for age and sex, because these are known risk factors for OA. BMI is also a known risk factor for OA and was different among the groups of knees based on meniscal damage (Table 1). However, because we suspected that BMI was potentially an important cause of abnormal loading which may have caused meniscal damage, subsequently causing a change in subchondral BMD, we did not adjust for BMI in our logistic regression model. On the other hand, we were not entirely certain of the causal pathway that exists in OA; therefore, we entertained the possibility that BMI was a confounder and subsequently adjusted for this additional factor in our logistic regression model. This factor was not significant in the model and the overall results remained similar.
Although we found that higher M:L BMD was associated with medial meniscal damage and lower M:L BMD was associated with lateral meniscal damage, we also found that maximal medial meniscal score and maximal lateral meniscal score were correlated. Those who have meniscal damage in one compartment are more likely to have meniscal damage in the other compartment as well. Longitudinal studies following those knees with meniscal damage in just one compartment will be critical in providing insight into this complex inter-relationship between meniscal damage and M:L BMD.
In our study, we also found that medial meniscal damage occurs 2.5 times more frequently than lateral meniscal damage (28% v. 11%). This may relate to the fact that 70% of the load within a knee is transmitted through the medial tibiofemoral compartment.27
These issues highlight the unique position of menisci. Not only do they serve the purpose of distributing load, but they are also the subject of loading within the knee. If meniscal damage occurs secondary to excess unicompartmental loading within the knee, then that same meniscus is also likely less able to distribute load within its compartment.
In our study we used a measurement of bone, the M:L BMD of the tibial plateau, a newly established feature of OA10 that has been associated with loading.11–13 Although this is not a direct measurement of loading, the fact that this measure, representing changes within the bone, was found to be associated with meniscal damage is of interest in better understanding OA pathophysiology. The results of our study suggest that there is a close relationship between meniscal damage and bone changes in OA.
The best chance of having a large public health impact in OA will involve identifying early changes seen in OA so that we can develop and implement interventions that may halt or reverse these changes. Improving our understanding of early OA, including that meniscal damage is associated with M:L BMD may be instrumental in this effort. Despite the marked trends demonstrated on a population basis in this study, it is difficult to predict the M:L BMD of a given individual based on their meniscal damage status. As illustrated in Figures 1–3, those with both medial and lateral meniscal damage have a wide range of M:L BMDs and the median of the ratio is similar to those in people without any meniscal damage. This may mean that the M:L BMD will have a limited impact on clinical care until the probability of OA development or progression can be predicted more accurately on a personal level. However, understanding the relationship of M:L BMD to that of meniscal damage on a population level does still provide insight into our understanding of the pathophysiology of OA. Longitudinal studies will be needed in order to clarify the temporal relationship between meniscal damage and increased regional BMD.
In summary, meniscal damage is associated with increased regional tibial BMD in the same compartment. Our findings highlight the close relationship between meniscal integrity and regional tibial subchondral BMD.
Figure 4.
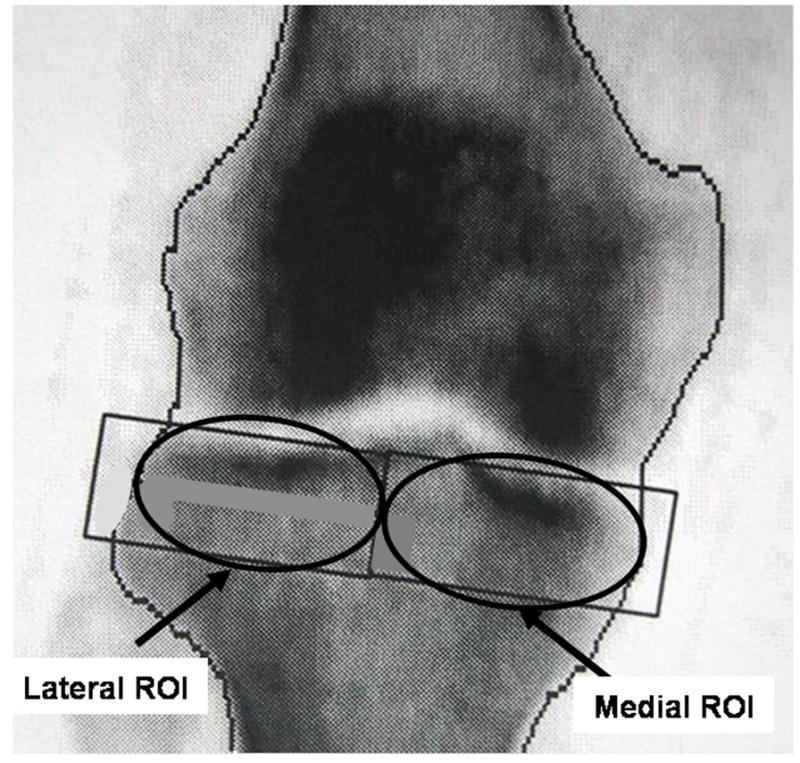
Diagram of the regions of interest used to calculate the M:L BMD Ratio.
Acknowledgments
We are indebted to Framingham subjects and staff of the Osteoarthritis Study. Without their help, this study would not have been possible.
Dr. Lo is supported by the American College of Rheumatology/Research and Education Foundation and the Arthritis Foundation through the Arthritis Investigator Award. Also, the Framingham Osteoarthritis Study is supported by NIH AR47785 and AG18393, the Framingham Osteoporosis Study is supported by NIH R01 AR/AG 41398, and the Framingham Heart Study by NIH contract N01-HC-25195 from the NHLBI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Voloshin AS, Wosk J. Shock absorption of meniscectomized and painful knees: a comparative in vivo study. J Biomed Eng. 1983;5(2):157–61. doi: 10.1016/0141-5425(83)90036-5. [DOI] [PubMed] [Google Scholar]
- 2.Fukubayashi T, Kurosawa H. The contact area and pressure distribution pattern of the knee. A study of normal and osteoarthrotic knee joints. Acta Orthop Scand. 1980;51(6):871–9. doi: 10.3109/17453678008990887. [DOI] [PubMed] [Google Scholar]
- 3.Cicuttini FM, Forbes A, Yuanyuan W, Rush G, Stuckey SL. Rate of knee cartilage loss after partial meniscectomy. J Rheumatol. 2002;29(9):1954–6. [PubMed] [Google Scholar]
- 4.Cooper C, McAlindon T, Snow S, Vines K, Young P, Kirwan J, et al. Mechanical and constitutional risk factors for symptomatic knee osteoarthritis: differences between medial tibiofemoral and patellofemoral disease. J Rheumatol. 1994;21(2):307–13. [PubMed] [Google Scholar]
- 5.Englund M, Roos EM, Lohmander LS. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum. 2003;48(8):2178–87. doi: 10.1002/art.11088. [DOI] [PubMed] [Google Scholar]
- 6.Roos EM, Ostenberg A, Roos H, Ekdahl C, Lohmander LS. Long-term outcome of meniscectomy: symptoms, function, and performance tests in patients with or without radiographic osteoarthritis compared to matched controls. Osteoarthritis Cartilage. 2001;9(4):316–24. doi: 10.1053/joca.2000.0391. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya T, Gale D, Dewire P, Totterman S, Gale ME, McLaughlin S, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg Am. 2003;85-A(1):4–9. doi: 10.2106/00004623-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54(3):795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 9.Lo GH, Hunter DJ, Zhang Y, McLennan CE, Lavalley MP, Kiel DP, et al. Bone marrow lesions in the knee are associated with increased local bone density. Arthritis Rheum. 2005;52(9):2814–21. doi: 10.1002/art.21290. [DOI] [PubMed] [Google Scholar]
- 10.Lo GH, Zhang Y, McLennan CE, Niu J, Kiel DP, McLean RR, et al. The Ratio of Medial to Lateral Tibial Plateau Bone Mineral Density and Compartment Specific Tibiofemoral Osteoarthritis. Osteoarthritis Cartilage. 2006;14:984–90. doi: 10.1016/j.joca.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Wada M, Maezawa Y, Baba H, Shimada S, Sasaki S, Nose Y. Relationships among bone mineral densities, static alignment and dynamic load in patients with medial compartment knee osteoarthritis. Rheumatology (Oxford) 2001;40(5):499–505. doi: 10.1093/rheumatology/40.5.499. [DOI] [PubMed] [Google Scholar]
- 12.Hulet C, Sabatier JP, Souquet D, Locker B, Marcelli C, Vielpeau C. Distribution of bone mineral density at the proximal tibia in knee osteoarthritis. Calcif Tissue Int. 2002;71(4):315–22. doi: 10.1007/s00223-001-2112-9. [DOI] [PubMed] [Google Scholar]
- 13.Hurwitz DE, Sumner DR, Andriacchi TP, Sugar DA. Dynamic knee loads during gait predict proximal tibial bone distribution. J Biomech. 1998;31(5):423–30. doi: 10.1016/s0021-9290(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 14.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. Jama. 2001;286(2):188–95. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 15.Chang A, Hayes K, Dunlop D, Hurwitz D, Song J, Cahue S, et al. Thrust during ambulation and the progression of knee osteoarthritis. Arthritis Rheum. 2004;50(12):3897–903. doi: 10.1002/art.20657. [DOI] [PubMed] [Google Scholar]
- 16.Sharma L, Dunlop D, Andriacchi TP, Hayes K, Song J, Cahue S, et al. The Adduction Moment and Knee Osteoarthritis (OA), a Longitudinal Study. Arthritis and Rheumatism. 2003;48(9 Supplement) Abstract #1131. [Google Scholar]
- 17.Akamatsu Y, Koshino T, Saito T, Wada J. Changes in osteosclerosis of the osteoarthritic knee after high tibial osteotomy. Clin Orthop. 1997;(334):207–14. [PubMed] [Google Scholar]
- 18.Katsuragawa Y, Fukui N, Nakamura K. Change of bone mineral density with valgus knee bracing. Int Orthop. 1999;23(3):164–7. doi: 10.1007/s002640050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30(8):914–8. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 20.Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5(4):297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- 21.Cronk CE, Roche AF. Race- and sex-specific reference data for triceps and subscapular skinfolds and weight/stature. Am J Clin Nutr. 1982;35(2):347–54. doi: 10.1093/ajcn/35.2.347. [DOI] [PubMed] [Google Scholar]
- 22.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Carbone LD, Nevitt MC, Wildy K, Barrow KD, Harris F, Felson D, et al. The relationship of antiresorptive drug use to structural findings and symptoms of knee osteoarthritis. Arthritis Rheum. 2004;50(11):3516–25. doi: 10.1002/art.20627. [DOI] [PubMed] [Google Scholar]
- 24.Kellgren JH, Lawrence JS. Radiologic assessment of osteoarthrosis. Annals of Rheumatic Disease. 1957;16:494–501. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheffe H. A method for judging all contrasts in the analysis of variance. Biometrika. 1953;40:87–104. [Google Scholar]
- 26.Zhang Y, Glynn RJ, Felson DT. Musculoskeletal disease research: should we analyze the joint or the person? J Rheumatol. 1996;23(7):1130–4. [PubMed] [Google Scholar]
- 27.Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975;(109):184–92. doi: 10.1097/00003086-197506000-00027. [DOI] [PubMed] [Google Scholar]