Abstract
The properties of non-selective cation channels were studied in rabbit portal vein smooth muscle cells in K+-free conditions with patch pipette techniques.
In about 45 % of isolated outside-out patches spontaneous channel currents with a unitary conductance of 23 pS were observed. The reversal potential was +11 mV which was shifted to more negative values and the unitary conductance was reduced when part of the external Na+ was replaced by Tris.
At negative potentials the probability of opening (Po) was low and the open time distributions were described by two exponentials with time constants of about 1 and 7 ms. At positive potentials Po and the longer mean open time were greatly increased.
The channel exhibited bursting behaviour and the burst duration distributions were described by two exponentials with time constants of about 3 and 15 ms. At positive potentials the longer burst duration was increased substantially.
Application of noradrenaline and the diacylglycerol analogue 1-oleoyl-2-acetyl-sn-glycerol (OAG) to the external membrane of quiescent patches evoked single-channel activity with a unitary conductance of about 23 pS and with similar kinetic behaviour to that of the spontaneous channel currents.
In conclusion, noradrenaline and OAG activate non-selective cation channel currents in excised patches of rabbit portal vein myocytes with a unitary conductance and kinetic properties that are similar to those of spontaneous channel currents. These channels have two open states and exhibit bursting behaviour. It is suggested that these channels underlie the whole-cell currents evoked by noradrenaline and OAG.
In rabbit portal vein smooth muscle cells noradrenaline acts on α1-adrenoceptors to evoke Ca2+-activated Cl− and K+ conductances and a non-selective cation current (Icat; Byrne & Large, 1988). The proposed physiological role of Icat is to produce membrane depolarisation with subsequent opening of voltage-dependent Ca2+ channels (VDCCs) to produce contraction of vascular smooth muscle. In addition it has been shown that the non-selective cation conductance is highly permeable to divalent cations (Byrne & Large, 1988; Wang & Large, 1991) and therefore Icat represents a pathway for the direct influx of Ca2+ ions to produce contraction independently of VDCCs.
It appears that an interesting transduction mechanism links the α1-adrenoceptor to the non-selective cation channel. Previous studies show that the α1-adrenoceptor is coupled to a G-protein, which activates phospholipase C to produce 1, 2-diacyl-sn-glycerol (DAG) to evoke Icat. Moreover, DAG activates Icat by a mechanism that is independent of protein kinase C (PKC; Helliwell & Large, 1997). More recently it has been proposed that either myosin light chain kinase (MLCK) or a MLCK isoform (or at least a kinase with similar pharmacological properties to MLCK) is also involved in the cascade, downstream of DAG, linking the receptor to the ionic channel (Aromolaran et al. 2000). In addition it appears that tyrosine kinase activation also elicits Icat (Albert et al. 2001). Consequently several transduction mechanisms converge on Icat, which indicates that this conductance may possess a role(s) other than simply being involved in contraction of vascular smooth muscle cells.
In earlier work we carried out fluctuation analysis of Icat recorded with whole-cell recording to ascertain some of the basic characteristics of the unitary conductance underlying Icat. From these studies the estimated single-channel conductance was 20-25 pS. Moreover, the spectral density function of Icat could be described by the sum of two Lorentzians, suggesting that the channel exists in at least three states (Helliwell & Large, 1998; Aromolaran & Large, 1999). However, more precise information on channel behaviour is obtained from studying the single channels directly. Some preliminary data on channels evoked by phenylephrine and acetylcholine in rabbit portal vein myocytes were described by Inoue & Kuriyama (1993). In the present paper, using isolated outside-out patches of rabbit portal vein smooth muscle cells, we describe in detail the properties of spontaneous non-selective cation channel currents. Moreover, these channels appear to be the same as those activated by noradrenaline and the DAG analogue 1-oleoyl-2-acetyl-sn-glycerol (OAG) in quiescent patches. Some of this work has been described in preliminary form (Albert & Large, 2000).
METHODS
Cell isolation
New Zealand White rabbits (2-3 kg) were killed by an i.v. injection of sodium pentobarbitone (120 mg kg−1) and the portal vein was removed and placed in normal physiological salt solution (PSS). The tissue was dissected free of connective tissue and fat before being cut into strips and placed in ‘Ca2+-free’ PSS. The tissue was enzymatically dispersed in two sequential enzyme steps. First, the strips of tissue were incubated in Ca2+-free PSS with 0.3 mg ml−1 protease Type XIV (Sigma) or protease Type VIII (Sigma) for 5 min and then the strips were washed in Ca2+-free PSS. In the second step the strips were incubated with 1 mg ml−1 collagenase Type 1A (Sigma) or collagenase Type IV (Sigma) in 100 μM Ca2+-PSS for 10 min and were then washed in 100 μM Ca2+-PSS. All enzyme and wash procedures were carried out at 37°C. After the enzyme treatments the strips were incubated in 100 μM Ca2+-PSS at room temperature (20-25°C) for 10 min before the cells were released into the solution by gentle mechanical agitation of the strips of tissue using a wide-bore Pasteur pipette. The suspension of cells was then centrifuged (1000 r.p.m.) to form a loose pellet that was resuspended in 0.75 mM Ca2+-PSS. The cells were then plated onto glass coverslips and stored at 4°C before use (1-6 h).
The normal PSS contained (mM): 126 NaCl, 6 KCl, 1.5 CaCl2, 1.2 MgCl2, 10 glucose and 11 Hepes; pH was adjusted to 7.2 with 10 M NaOH. Ca2+-free PSS, 100 μM Ca2+-PSS and 0.75 mM Ca2+-PSS had the same composition except that either Ca2+ was omitted or 1.5 mM CaCl2 was replaced by 100 μM CaCl2 and 0.75 mM CaCl2, respectively.
Electrophysiology
Single non-selective cation channel currents were recorded with a List L/M-PC patch-clamp amplifier at room temperature using the standard excised outside-out patch configuration (Hamill et al. 1981). Patch pipettes were manufactured from borosilicate glass and were routinely coated in Sylgard (Dow Corning, Germany) to reduce stray capacitance and fire polished to increase seal resistance, giving pipette resistances between 6 and 10 MΩ when filled with the standard internal patch pipette solution. The junction potential between the pipette solution and the external solution was calculated by measuring the change in voltage on replacement of the external solution with the pipette solution. The change in voltage was found to be < 3 mV and was not compensated in the final records. Series resistance was not compensated. To reduce ‘line’ noise the recording chamber (volume ca 150-200 μl) was perfused using two 10 ml syringes, one filled with external solution and the other used to drain the chamber, in a ‘push and pull’ technique. The external solution could be exchanged twice within 20 s. This method of solution exchange caused small changes in the holding current, and single-channel currents that were activated by noradrenaline, for example, were analysed when the baseline current had settled down (within a few seconds of solution change). All experiments were initially carried out at a holding potential of -50 mV. To evaluate the unitary single cation channel current- voltage (I-V) characteristics, the membrane potential was manually stepped from -50 mV to between -90 and +40 mV.
Single-channel currents were initially recorded onto digital audiotape (DAT) using a CDATA digital tape-recorder (Cygnus Technology Inc., Delaware, PA, USA) at a bandwidth of 5 kHz (-3 db, low-pass 4-pole Bessel filter, List L/M-PC patch-clamp amplifier) and a sample rate of 48 kHz. For off-line analysis single-channel current records were filtered at 1 kHz (-3 db, low-pass 8-pole Bessel filter, Frequency Devices, model LP02, Scensys Ltd, Aylesbury, UK) and acquired using a CED 1401plus interface and CED Patch and Voltage Clamp Software (version 6.0, Cambridge Electronic Design Ltd, Cambridge, UK) at a sampling rate of 10 kHz. Data were filtered at 1 kHz since this filter setting has a maxmium resolution of 0.664 ms (2 times the rise time of a 1 kHz filter) and can resolve over 90 % of the single ion channel amplitude (Colquhoun, 1987). Data were captured with a Pentium (P5-100) personal computer (Gateway, Ireland).
Mean single cation channel amplitudes were calculated from idealised traces of at least 10 s in duration with a stable baseline using the half-amplitude crossing method. I-V relationships were calculated in two ways. First, the single cation channel amplitudes from an individual patch were plotted and the unitary conductance was calculated from the slope of the relationship using linear regression (Origin software, Microcal, USA). The reversal potential (Erev) was calculated directly from the I-V relationship by interpolation. In some experiments Erev was calculated by extrapolation and these values were similar to those obtained by interpolation. The unitary conductance and Erev from all the individual patches tested were then used to calculate mean conductance and mean Erev values. Second, in some experiments single cation channel amplitudes from individual patches at different membrane potentials were pooled together and the unitary conductance and Erev were determined by linear regression. Lifetime distributions were plotted using a 1 ms bin width and where appropriate were fitted with one or more exponential functions using the maximum likelihood method. Events that lasted for < 0.664 ms were excluded from analysis. Po was calculated using the equation:
Burst duration analysis was carried out on single cation channel currents with more than one closed time constant, with the value of the fast time constant being at least 20 times smaller than any other time constant. Similar to the method of Magleby & Pallotta (1983), the critical time value (Tcrit) used to distinguish closures within a burst from closures between bursts was calculated as 3-4 times the fast closed time constant. Figure preparation was carried out using Origin software.
Solutions and drugs
The cells were perfused in a standard K+-free external solution containing (mM): 126 NaCl, 1.5 CaCl2, 11 Hepes and 10 glucose; pH 7.2 with 10 M NaOH. The standard internal patch pipette solution contained (mM): 18 CsCl, 108 caesium aspartate, 1.2 MgCl2, 10 Hepes, 11 glucose, 10 BAPTA and 1 CaCl2 (free internal calcium ([Ca2+]i) approximately 14 nM, as calculated using EQCAL software); pH 7.2 with Tris. Under these conditions voltage-gated Ca2+ currents, K+ currents and Ca2+-activated conductances are abolished at the holding potential of -50 mV and single non-selective cation channel currents could be recorded in isolation. Moreover, with the anion gradient the chloride equilibrium potential (ECl) is about -50 mV, i.e. the holding potential. Propranolol (1 μM) was added to all solutions containing noradrenaline to block β-adrenoceptor activation. In cell-attached experiments the bath and patch pipette solution used was the standard K+- free solution. The cell-attached patch pipette solution also contained 10 mM TEA, 5 mM 4-AP, 100 μM niflumic acid, 100 μM DIDS and 3 μM nicardipine to prevent contamination from K+, Ca2+-activated Cl−, swell-activated Cl− and voltage-dependent Ca2+ currents. All drugs were purchased from Sigma (UK). The values are the means of n cells ±s.e.m. Statistical analysis was carried out using Student's t test, with the level of significance set at P < 0.05.
RESULTS
Spontaneous non-selective cation channels in outside-out patches
In 1.5 mM [Ca2+]o 112/250 excised outside-out patches exhibited spontaneous single cation channel currents. Figure 1 shows a typical experiment where the excision of an outside-out patch from the whole-cell configuration caused the appearance of spontaneous single inward cation channel currents at the holding potential of -50 mV. Figure 1A shows the transition between whole-cell recording and the excision of an outside-out patch recorded on a slow time scale. Figure 1B illustrates that the record of the whole-cell membrane current did not contain any fluctuations suggestive of single cation channel activity at -50 mV. However, when an excised outside-out cell membrane patch was removed from the whole-cell configeration, single inward cation channel activity was readily observed at the same potential (Fig. 1C). In Fig. 1D, the gain of the patch-clamp amplifier was increased so that the properties of the single-channel currents could be observed. One out of 31 cell-attached patches tested also contained spontaneous single ion channel currents at patch potentials between +100 and -100 mV.
Figure 1. Excision of an outside-out patch reveals spontaneous non-selective cation channels.
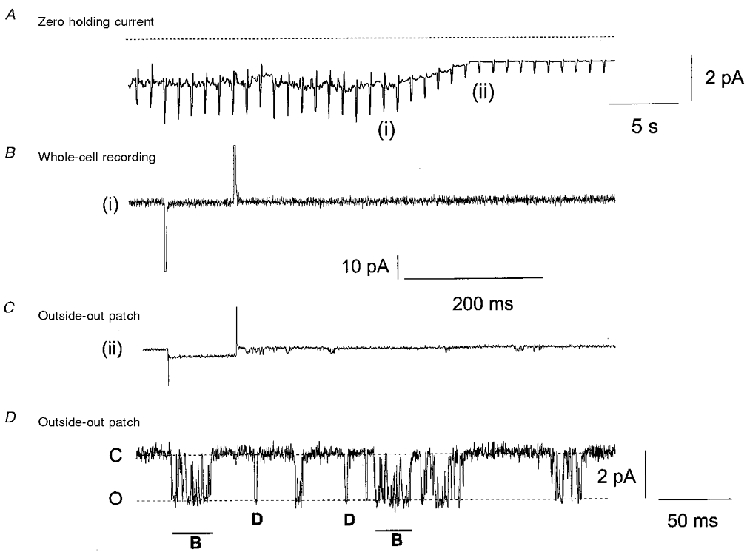
A shows that excision of an outside-out patch between position (i) in whole-cell recording and (ii) excised outside-out patch reduces both holding current and ‘noise’. The rapid deflections in membrane current represent responses to a 10 mV hyperpolarising pulse. B shows the whole-cell current immediately prior to excision of the outside-out patch, (i) in A, and there was no distinctive single-channel activity. C illustrates that immediately after excision, (ii) in A, single inward ion channels (represented as downward deflections) were observed at the holding potential of -50 mV. D shows that the single ion channels displayed on a higher gain either opened for brief discrete periods of time (D) or displayed bursting characteristics (B). C, closed; O, open.
In all 112 patches the spontaneous single cation channel activity was observed immediately (within 1 s) after excision of the outside-out patch from the whole-cell configuration. In the remaining 138 outside-out patches, which did not show spontaneous single cation channel activity immediately after excision, single-channel openings did not develop over the following 5-10 min. In some outside-out patches spontaneous single-channel activity declined after approximately 2-3 min, but in many patches the activity was sustained for over 20 min, enabling the study of the properties of the spontaneous single-channel currents.
The single inward cation channel currents had peak amplitudes of between 1 and 2 pA at -50 mV and the channels had a distinctive pattern of opening (Fig. 1D). The single inward cation channels either opened for brief discrete periods of time (Fig. 1D, D) before they closed or opened for a longer time but during this period of opening the cation channel flickered between the open and closed states (Fig. 1D, B). The pattern of flickering closures during the opening of ion channels is characteristic of an ion channel exhibiting bursting behaviour (Colquhoun & Hawkes, 1995).
The effect of patch potential on spontaneous single cation channel current amplitudes
Figure 2A illustrates spontaneous single cation channel currents recorded from the same outside-out patch when the membrane potential was changed from -50 to + 40 mV. Figure 2B shows single cation channel amplitude histograms created from individual events recorded from the outside-out patch shown in Fig. 2A. Figure 2C illustrates the I-V relationship calculated from the mean peak current amplitudes shown in Fig. 2B The I-V relationship behaved ohmically between -50 and +40 mV and had a unitary slope conductance of 24 pS and a reversal potential (Erev) of +10 mV. In 17 outside-out patches the mean unitary conductance was 23 ± 0.7 pS and the mean Erev was +11 ± 0.6 mV.
Figure 2. The effect of membrane potential on single cation channel amplitude.
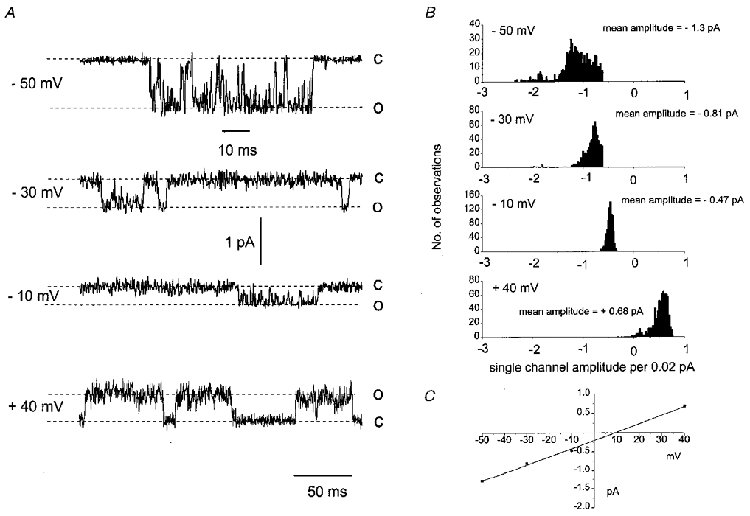
A shows that changing membrane potential from -50 to -10 mV reduced the amplitude of the single inward cation channels recorded from the same patch. At +40 mV the single cation channels opened in the outward direction. Also note that at +40 mV the channels spent more time in the open state. B illustrates the single ion channel histograms from the same patch shown in A. Note that at -50 and -30 mV the histograms show a small number of double openings, suggesting two channels in the patch. C, the current-voltage relationship plotted from the mean single-channel amplitudes had a slope conductance of 24 pS and a Erev of +10 mV.
The effect of patch potential on Po
Figure 2A shows that the single cation channel appeared to spend a greater period of time in the open state at positive membrane potentials compared with negative potentials. We investigated this observation further by comparing the effect of patch potential on Po. Table 1 shows that between -90 and -10 mV, Po was low (about 0.05) and was not significantly different, whereas at +40 mV, Po was increased more than 10-fold (Table 1).
Table 1.
The effect of membrane potential on Po and open lifetime distributions of single spontaneous non-selective cation channels
| Membrane potential (mV) | Oτ1 (ms) | Oτ2 (ms) | Po | n |
|---|---|---|---|---|
| −90 | 1.2 ± 0.1 | 5.0 ± 0.7 | 0.03 ± 0.01 | 4 |
| −70 | 1.0 ± 0.1 | 6.9 ± 0.1 | 0.05 ± 0.01 | 4 |
| −50 | 1.1 ± 0.1 | 7.7 ± 0.4 | 0.04 ± 0.03 | 31 |
| −30 | 1.1 ± 0.1 | 8.3 ± 0.9 | 0.05 ± 0.02 | 4 |
| −10 | 1.0 ± 0.1 | 6.8 ± 0.9 | 0.03 ± 0.01 | 4 |
| +40 | 1.5 ± 0.2 | 40 ± 7.5* | 0.62 ± 0.15* | 5 |
P < 0.001 compared with the values at −50 mV.
Replacement of external Na+ ions by Tris ions
With K+-free conditions and with ECl set at about -50 mV the Erev value of +10 mV suggests that the spontaneous single-channel currents are non-selective cation channels. We tested this possibility by studying the effect of replacing part of the external Na+ ions (63 mM NaCl) with the less permeant cation Tris (63 mM TrisCl) on the unitary slope conductance and Erev of the spontaneous single ion channel currents. Figure 3 shows the pooled data from several patches in which the single cation channel amplitudes were recorded at different membrane potentials. In 126 mM NaCl the pooled I-V relationship was similar to the I-V relationship of single cation channel currents recorded from an individual outside-out patch shown in Fig. 2D. The I-V relationship behaved ohmically between -90 and +40 mV, with a unitary slope conductance of 25 pS and Erev of +10 mV.
Figure 3. The effect of replacing Na+ ions with Tris ions.
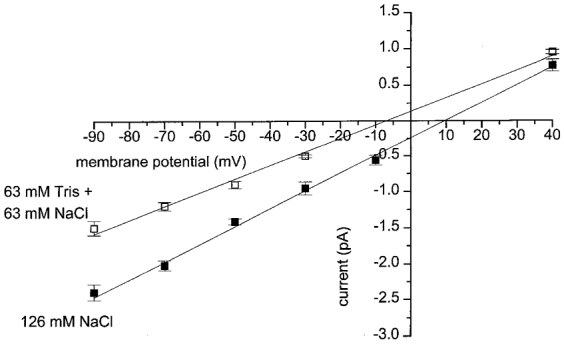
Current-voltage relationships created from pooled data show that replacing 63 mM NaCl with 63 mM TrisCl reduced slope conductance from 25 pS (▪) to 19 pS (□) and shifted the reversal potential from +10 to -7 mV. Each point is the mean of between 4 and 18 patches.
In the presence of 63 mM NaCl/63 mM TrisCl, the I-V relationship of the spontaneous single cation channel currents was also linear between -90 and +40 mV. However, replacing 63 mM NaCl with 63 mM TrisCl reduced the unitary slope conductance to 19 pS and shifted the Erev to -7 mV. The reduction in unitary conductance and the shift in Erev in the presence of the less permeant Tris ion provides further evidence that the spontaneous single ion channel currents in rabbit portal vein cells result from opening of non-selective cation channels.
Kinetics of single cation channel activity
The effect of patch potential on the open time distributions of the single-channel currents
Figure 4A illustrates the open lifetime distribution compiled from spontaneous single cation channel currents in one outside-out patch recorded at -50 mV. The majority of the openings were brief (< 3 ms) although there were a significant number of openings that were greater than 5 ms. The open lifetime distribution could be fitted by the sum of two exponentials using the maximum likelihood method with time constants of 1.2 ms (Oτ1) and 7 ms (Oτ2), suggesting that the non-selective cation channels have at least two open states. In 31 patches at -50 mV, Oτ1 was 1.1 ± 0.1 ms and Oτ2 was 7.7 ± 0.4 ms (n = 31, Table 1).
Figure 4. The effect of membrane potential on open time distributions.
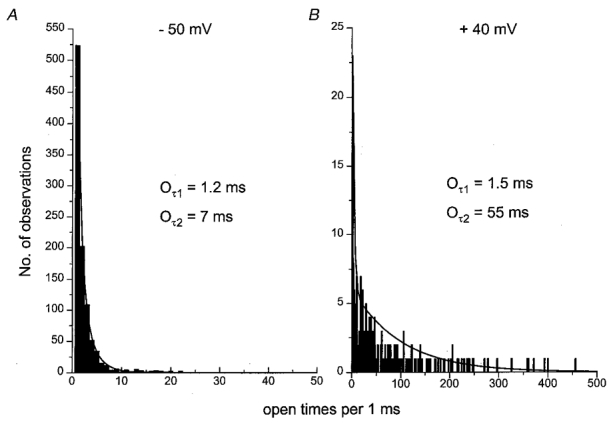
A, at -50 mV the open time distribution could be described by the sum of two time constants of 1.2 ms (Oτ1) and 7 ms (Oτ2). B, in the same patch the slower time constant (Oτ2) was increased to 55 ms at +40 mV.
It was found that at all the potentials studied the open lifetime distribution could be fitted by two exponentials and Table 1 shows that between -90 and -10 mV the time constants Oτ1 and Oτ2 were not significantly different (P > 0.05). However, at +40 mV, although Oτ1 was similar to the corresponding values at negative potentials, the time constant Oτ2 was greatly increased. Figure 4B shows the distribution of open times at +40 mV in the same patch as illustrated in Fig. 4A. It can be seen that at +40 mV, Oτ1 was similar but the longer time constant (Oτ2= 55 ms) was about 8 times greater than the corresponding value at -50 mV (see also Table 1). The increase in the open time distribution at +40 mV reflects the longer openings observed at this potential compared with more negative membrane potentials (e.g. see Fig. 2A).
The effect of membrane potential on the closed time distributions of the single-channel currents
In Figs 1D and 2A the spontaneous cation channel activity appeared to exhibit bursting behaviour and we attempted to gain some insight into the kinetics of this bursting activity of the non-selective cation channel currents. In order to achieve this aim it is necessary to analyse the closed time distributions. However, it should be pointed out that in this study we make no claim regarding the number of closed states of the channel, since with such a low Po it is not possible to be certain that there is a single-channel in the patch, which is a necessary criterion for making conclusions about the number of closed states from the closed time distribution.
Figure 5A shows a typical closed lifetime distribution of spontaneous cation channel currents at -50 mV. The closed lifetime distribution could be described by the sum of two exponentials with a time constant Cτ1 of 1.9 ms and a time constant Cτ2 of 160 ms. The large difference between the two closed time constants is indicative of an ion channel behaving in a bursting manner, with the brief closures (Cτ1) representing the closed times within bursts and the longer closed time (Cτ2) representing closures between bursts of cation channel activity. All the spontaneous single cation channel currents recorded at -50 mV had closed lifetime distributions that could be described by the sum of two exponentials; mean Cτ1 was 2.1 ± 0.2 ms (n = 24) and mean Cτ2 was 162 ± 25 ms (n = 24, Table 2). Table 2 shows that at all membrane potentials the closed time distribution histograms could be fitted by the sum of two exponentials, and that between -90 and -10 mV the two closed time constants (Cτ1 and Cτ2) were similar. However, at +40 mV, whereas Cτ1 was similar to the values at negative potentials, Cτ2 was found to be significantly reduced (Fig. 5B, Table 2).
Figure 5. The effect of membrane potential on closed time distribution and burst durations.
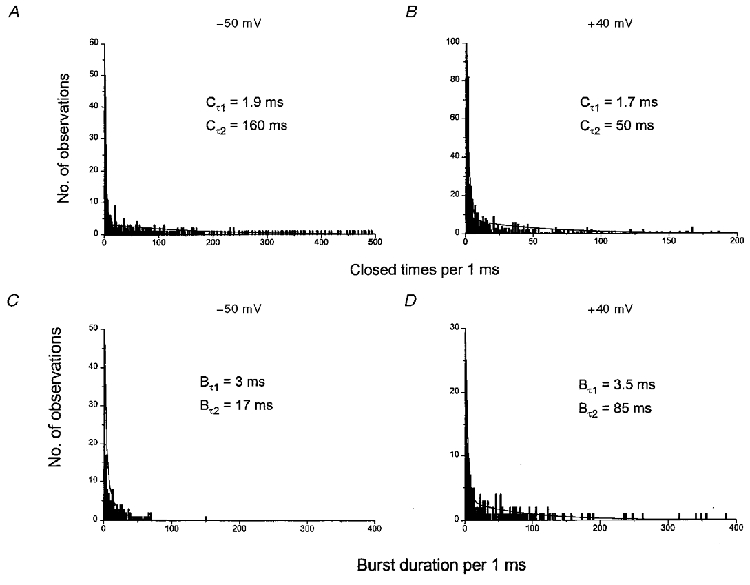
A and B, distribution of closed times at -50 and +40 mV, respectively. C and D, distribution of burst durations at -50 and +40 mV, respectively.
Table 2.
The effect of membrane potential on closed lifetime distributions and burst duration of single spontaneous nonselective cation channels
| Membrane potential (mV) | Cτ1 (ms) | Cτ2 (ms) | Bτ1 (ms) | Bτ2 (ms) | n |
|---|---|---|---|---|---|
| −90 | 2.7 ± 0.2 | 120 ± 17 | 3.3 ± 0.3 | 13 ± 2.4 | 4 |
| −70 | 2.4 ± 0.4 | 113 ± 21 | 2.8 ± 0.4 | 13 ± 1 | 4 |
| −50 | 2.1 ± 0.2 | 162 ± 25 | 3.5 ± 0.3 | 16 ± 1.9 | 24 |
| −30 | 2.0 ± 0.6 | 120 ± 15 | 3.3 ± 0.3 | 16 ± 1.5 | 4 |
| −10 | 1.4 ± 0.5 | 158 ± 18 | 3.0 ± 0.2 | 14 ± 0.7 | 4 |
| +40 | 1.8 ± 0.1 | 80 ± 21** | 3.8 ± 0.5 | 104 ± 19* | 5 |
P < 0.05
P < 0.01 compared with the values at −50 mV.
The effect of membrane potential on the burst duration distributions of the single-channel currents
To calculate burst duration distribution a closed time must be determined that is the threshold level for closures within bursts and between bursts. This threshold level is called the critical time (Tcrit) and any closed time below this level is determined as a closure within a burst, and a closed time greater than this value is a closure between bursts. In our experiments Tcrit was determined from the faster closed time constant (Cτ1, see Methods). The mean Tcrit used in our studies was 6.5 ± 0.4 ms (n = 24).
Figure 5C illustrates a typical burst duration histogram calculated from the closed times of the spontaneous cation channel currents shown in Fig. 5A. The burst duration histogram could be described by the sum of two exponentials with a time constant Bτ1 of 3 ms and a time constant Bτ2 of 17 ms. This suggests that the spontaneous channel activity in this patch exhibited either a brief bursting behaviour which lasted for 3 ms or longer burst durations which lasted for 17 ms. In 24 patches tested, the shorter mean burst duration (Bτ1) was 3.5 ± 0.3 ms and the longer mean burst duration (Bτ2) was 16 ± 1.9 ms (Table 2).
Table 2 illustrates that at all potentials the burst duration histograms could all be fitted by two exponentials and that between -90 and -10 mV the two time constants (Bτ1 and Bτ2) had similar values. However, at +40 mV, although Bτ1 was not significantly altered, the burst duration Bτ2 was significantly increased approximately 5- to 7-fold to 104 ms (Fig. 5D, Table 2).
Noradrenaline activates single ion channel currents in outside-out patches with properties similar to those of the spontaneous non-selective cation channel currents
The single-channel conductance and the kinetic behaviour of the spontaneous channel currents described in the present study are similar in some respects to the properties of the unitary conductance activated by noradrenaline estimated using ‘noise’ analysis in previous studies (Helliwell & Large, 1998; Aromolaran & Large, 1999; Albert et al. 2001). Therefore we investigated whether noradrenaline could activate single cation channel activity in outside-out patches with similar properties to those of the spontaneous cation channels.
In 10/93 excised outside-out patches, in which there was no spontaneous activity, application of 1-100 μM noradrenaline activated inward single ion channel currents at the holding potential of -50 mV. None of the ten patches showed any spontaneous single cation channel activity for at least 5 min following excision before noradrenaline was applied. Noradrenaline-activated single cation channel currents had similar appearances to spontaneous single cation channel currents with peak amplitudes between 1 and 2 pA at -50 mV, and an appearance of either brief openings or openings containing flickerings to the closed level characteristic of bursting behaviour. Single-channel activity was evoked immediately (within a few seconds) after application of noradrenaline, although with this method of drug application it was not possible to obtain a precise value of the latency of channel activation to noradrenaline. In six of the ten patches channel activity was well sustained for 5-10 min, but in four of the patches channel activity declined after 1-2 min. Figure 6A illustrates a typical response of an outside-out patch to an application of 1 μM noradrenaline. In this outside-out patch, similar to the properties of spontaneous single cation channels, the noradrenaline-evoked cation channel currents had a unitary conductance of 25 pS and Erev of +11 mV (e.g. see Fig. 6B). Moreover, open lifetime distributions were described by the sum of two exponentials with time constants of 1.7 and 7 ms (Fig. 6C). Also the burst duration distribution was described by the sum of two exponentials with time constants of 3.7 and 17 ms (Fig. 6D). In six outside-out patches the mean single-channel conductance of the noradrenaline-activated inward cation channel currents was 23 ± 1.5 pS and the mean Erev was +11 ± 2 mV (Table 3). Mean time constants of the open time distributions were 1.4 ± 0.4 ms (Oτ1) and 6.3 ± 1.4 ms (Oτ2, n = 6), and mean burst duration time constants were 3.2 ± 0.8 ms (Bτ1) and 16 ± 1.4 ms (Bτ2, n = 6, Table 3). In addition, Po of the noradrenaline-evoked single inward ion channel currents was 0.05 ± 0.02 (n = 10) at -50 mV. All of these values of the noradrenaline-evoked channel currents were similar to the values associated with the spontaneous non-selective cation channel activity.
Figure 6. Noradrenaline activates non-selective cation channels in outside-out patches.
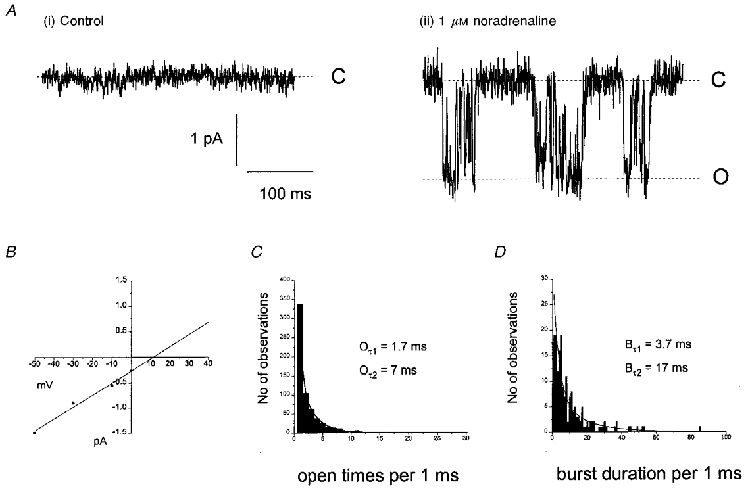
A, in an outside-out patch that did not contain any spontaneous activity (i), application of 1 μM noradrenaline activated single cation channels (ii). B, current-voltage relationship plotted from the single cation channel amplitudes recorded from the same patch as A, which had a slope conductance of 25 pS and a reversal potential of +11 mV. The open time (C) and burst duration (D) distributions calculated from the cation channels recorded in A were similar to those calculated for the spontaneous cation channels.
Table 3.
Comparison of single-channel data with the characteristics of the unitary conductance estimated with spectral density function analysis of the whole-cell current to noradrenaline
| γ (pS) | Erev (mV) | τ1 (ms) | τ2 (ms) | Bτ1 (ms) | Bτ2 (ms) | |
|---|---|---|---|---|---|---|
| Spontaneous cation channels | 23 ± 1 | +10 ± 1 | 1.1 ± 0.1† | 7.7 ± 0.4† | 2.5 ± 0.3 | 15 ± 1.9 |
| Noradrenaline-evoked cation channels | 23 ± 2 | +11 ± 2 | 1.4 ± 0.4† | 6.3 ± 1.4† | 3.2 ± 0.8 | 16 ± 1.4 |
| OAG-evoked cation channels | 24 ± 2 | +8 ± 2 | 1.1 ± 0.2† | 5.7 ± 2.2† | 3.3 ± 0.2 | 17 ± 1.5 |
| Noradrenaline-evoked Icat‡ | 19 ± 2 | +9 ± 1 | 1.6 ± 0.3* | 15 ± 1* | — | — |
These values are represented as Oτ and Oτ2 in Table 1.
Data estimated from spectral density function analysis.
These values were obtained from ±=½2πfc, where fc is the corner frequency (see Helliwell & Large, 1998; Albert et al. 2001).
The diacylglycerol analogue 1-oleoyl-2-acetyl-sn-glycerol also activates single ion channel currents in outside-out patches
Previous studies have shown that 1-oleoyl-2-acetyl-sn-glycerol (OAG), a DAG analogue, can activate Icat via a PKC-independent mechanism (Helliwell & Large, 1997). Therefore we investigated whether application of OAG could activate single non-selective cation channel currents in outside-out patches with similar properties to those of both the spontaneous and noradrenaline-evoked cation channel currents.
In 5/39 outside-out patches, application of 10-100 μM OAG activated inward single cation channel currents at -50 mV. As in the above experiments with noradrenaline we did not apply OAG unless the patches did not contain any spontaneous events for at least 5 min following excision. Figure 7A shows a typical response of an outside-out patch to an application of 10 μM OAG. The single cation channel activity had a similar bursting appearance to that of spontaneous and noradrenaline-activated cation channel currents (Fig. 7A (ii)). As with noradrenaline OAG evoked single-channel activity within the time of solution exchange (20 s) and single-channel activity was sustained for 5-10 min in all five patches. In this patch the OAG-activated cation channel currents had a slope conductance of 23 pS and Erev of +10 mV (Fig. 7B) with an open time distribution described by the sum of two exponentials with time constants of 1.4 and 6 ms (Fig. 7C). The burst duration for this patch could also be described by the sum of two expotentials with time constants of 3.5 and 20 ms (Fig. 7D). In five outside-out patches the mean single cation channel conductance was 24 ± 2 pS and the mean Erev was +8 ± 2 mV (Table 3). The mean time constants of the open time distributions were 1.1 ± 0.2 and 5.7 ± 2.2 ms (n = 5) and the mean burst duration time constants were 3.3 ± 0.2 and 17 ± 1.5 ms (n = 5, Table 3) at -50 mV. In addition, Po of the OAG-activated cation channel currents was 0.02 ± 0.01 (n = 5) at -50 mV. These properties of the OAG-activated cation channel currents are similar to the conductance and kinetics values of both the spontaneous and noradrenaline-evoked cation channel currents.
Figure 7. The diacylglycerol analogue OAG activates non-selective cation channels with similar characteristics to those of the spontaneous and noradrenaline-activated cation channels.
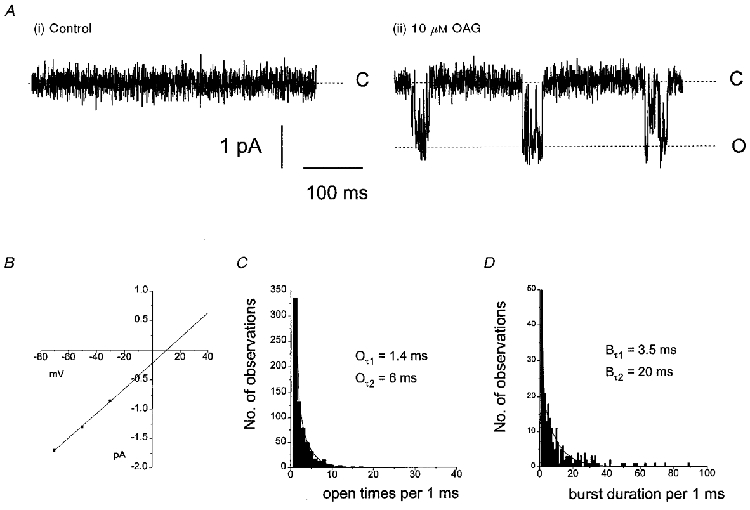
A shows that application of 10 μM OAG activates single cation channels (ii) in an outside-out patch which previously did not contain any spontaneous events (i). B, from the same patch the current-voltage relationship had a slope conductance of 23 pS and a reversal potential of +10 mV. The open time (C) and burst duration (D) distributions were similar to those for spontaneous and noradrenaline-evoked cation channels.
DISCUSSION
Similar properties of spontaneous non-selective cation channel currents to those evoked by noradrenaline and OAG
In the present work we describe the properties of spontaneous single non-selective cation channel currents and show that these are similar to channel currents evoked by noradrenaline and OAG in outside-out patches of rabbit portal vein smooth muscle cells. From Table 3 it can be seen that the single-channel conductance, reversal potential, mean open times and burst durations are similar for spontaneous, noradrenaline- and OAG-evoked channel currents. It is reasonable to conclude that it is the same channel observed in the three experimental conditions. Also in Table 3 we include the parameters estimated from analysis of the spectral density function of noradrenaline-evoked Icat recorded with whole-cell recording. It can be seen that noise analysis predicts the channel properties accurately in many respects. However, it should be noted that in noise analysis of the whole-cell current, whereas the time constant of the higher frequency Lorentzian represents the briefer mean channel open time, the time constant estimated from the second Lorentzian (τ2= 15 ms, Table 3) does not reflect the longer channel mean open time (Oτ2 of about 6-7 ms, Table 3). Instead this value reflects the longer of the two burst durations (Bτ2 of 15-17 ms). With ‘noise’ analysis of whole-cell Icat, the second Lorentzian contributes about 99 % to the overall variance (Helliwell & Large, 1998) and therefore it seems that the longer burst duration dominates the kinetics of the fluctuations of the whole-cell current.
The observation that noradrenaline and OAG activated the channel in isolated patches suggests that the transduction mechanism linking the α1-adrenoceptor to the channels is effectively membrane delimited. This is in agreement with the central role of DAG in opening the channels. Noradrenaline and OAG activated channels in a much smaller proportion of patches (about 10 %) than were observed with spontaneous channels (45 %). It is possible that many of the unresponsive patches did not contain channels since no spontaneous channels were observed in those patches. Consequently noradrenaline and OAG may be more effective in activating these channels in isolated patches than this statistic suggests. However, as outlined in the Introduction, a relatively complex pathway is involved in activation of the channels by noradrenaline and OAG and therefore it is also possible that this pathway is disrupted on excision of some patches. Also it is interesting that spontaneous channel currents were seen in a much higher proportion of isolated patches (about 45 %) than in cell-attached patches (about 3 %). It is also interesting that at negative potentials single-channel currents to noradrenaline were not detected with cell-attached patch recording. The simplest explanation for these observations is that excision of the patch from the membrane removes an inhibitory influence. The most likely source of this inhibiting modulator is the cytosol and thus there may be a cytosolic factor that suppresses the activity of this non-selective cation channel.
An interesting observation is that the Po of noradrenaline-evoked channel activity was low, about 0.05, but noise analysis of the whole-cell current also indicated that Po was low even when using maximal concentrations of noradrenaline (Helliwell & Large, 1998). If a kinase is involved in channel opening, a low Po may be explained by a very active dephosphorylation process (i.e. a very efficient phosphatase).
Voltage-dependent properties of non-selective cation channel currents
The relationship between single-channel amplitude and potential was linear between -90 and +40 mV. However, with whole-cell recording of Icat there is little outward current between Erev (+10 mV) and +40 mV (e.g. see Helliwell & Large, 1996). This suggests that in whole-cell recording there is a non-dialysable factor that inhibits outward current flow between +10 and +40 mV, and this regulation is removed on patch excision. Another curious phenomenon was the dependence of Po on voltage. At negative potentials Po was low (0.03-0.05) and was similar between -90 and -10 mV when current flow is inward (see Table 1). However, at +40 mV, when net current flows outward, Po was increased about 10-fold. There is no clear explanation for this observation, although it is possible that Po is to some extent dependent on the direction of net current flow across the membrane. For example, it is possible that external Ca2+ ions may block the inward current at negative potentials. Previously it has been shown that millimolar concentrations of Ca2+ ions inhibit the amplitude of noradrenaline-evoked whole-cell current (Helliwell & Large, 1996). Experiments are being carried out at present to test this possibility.
Kinetic properties of non-selective cation channel currents
The distribution of open times suggests that there are two open states with mean open times of about 1 and 7 ms. These estimates agree with the values of Inoue & Kuriyama (1993) for channels activated by phenylephrine in the same preparation. The distribution of closed times also indicates that there are two closed states, although caution must be used regarding this conclusion because there is no certainty that there was only one channel in the patch. Indeed at positive potentials when Po was relatively high, double openings could be seen in some patches. However, the mean closed time constants (about 2 and 120-160 ms) were so different that it is tempting to speculate that there are at least two closed states. In this case the presence of more than one channel in the patch would underestimate the longer closed time constant Cτ2.
It was evident that the channel exhibited bursting behaviour and that burst duration distributions were also described by two exponentials with time constants of about 3 and 15 ms. The mean open and closed times and burst durations were potential independent at negative potentials, but at +40 mV the longer mean open time Oτ2 increased about 6-fold and the longer closed time Cτ2 was significantly reduced (see Table 2). In addition the longer burst duration Bτ2 was markedly increased at +40 mV compared with negative potentials (Table 2). Therefore the increase in Oτ2 and Bτ2 associated with a reduction of Cτ2 accounts for the large increase in Po observed at positive compared with negative potentials.
Comparison with transient receptor potential and transient receptor potential-like channel currents
Although the molecular identity of the channel described in this paper is not known, it is becoming increasingly evident that there are several similarities between these channels and the transient receptor potential (TRP) and transient receptor potential-like (TRPL) ion channels originally described in Drosophila photoreceptors (see Harteneck et al. 2000). First, in rabbit portal vein smooth muscle cells the non-selective cation channels are activated by DAG in a PKC-independent manner (Helliwell & Large, 1997). It has also been shown that the mammalian homologue hTRPC6, hTRPC3 and mouse TRP7 are non-selective cation channels that are activated by DAG independently of PKC (Hofmann et al. 1999; Okada et al. 1999). Second, the relative permeability of some of the channels to divalent cations is similar. From reversal potential measurements in different cation solutions we estimated the relative permeability of Ba2+ to Na+ (PBa/PNa) in rabbit portal vein cells to be 4.6 (Wang & Large, 1991). In Drosophila photoreceptors PBa/PNa of TRPL currents was calculated to be 4.2 (Reuss et al. 1997). In the same study the unitary conductance of the TRPL conductance estimated by noise analysis was 35 pS (cf. our value of 20-25 pS in rabbit portal vein in the present study). Third, in the absence of activators channels open and close spontaneously in isolated patches of rabbit portal vein smooth muscle cells (present work) and also in patches containing TRP and TRPL channels (e.g. see Chyb et al. 1999; Hofmann et al. 1999). Finally, the probability of channel opening Po is greatly increased at positive potentials in portal vein (our study) and with TRP and TRPL channels (Chyb et al. 1999; Hofmann et al. 1999). Consequently it is possible that the non-selective cation channel described in the present work is part of the TRP or TRPL family of channels.
Acknowledgments
This work was supported by The British Heart Foundation. We are grateful to Dr R. M. Helliwell for carrying out the earlier experiments.
References
- Albert AP, Aromolaran AS, Large WA. Agents that increase tyrosine phosphorylation activate a non-selective cation current in single rabbit portal vein smooth muscle cells. The Journal of Physiology. 2001;530:207–217. doi: 10.1111/j.1469-7793.2001.0207l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Large WA. The effect of external calcium ions on spontaneous non-selective cation channels in rabbit portal vein myocytes. The Journal of Physiology. 2000;527.P:108P. doi: 10.1111/j.1469-7793.2001.0457k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aromolaran AS, Albert AP, Large WA. Evidence for myosin light chain kinase mediating noradrenaline-evoked cation current in rabbit portal vein myocytes. The Journal of Physiology. 2000;524:853–863. doi: 10.1111/j.1469-7793.2000.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aromolaran AS, Large WA. Comparison of the effects of divalent cations on the noradrenaline-evoked cation current in rabbit portal vein smooth muscle cells. The Journal of Physiology. 1999;520:771–782. doi: 10.1111/j.1469-7793.1999.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne NG, Large WA. Membrane ionic mechanisms activated by noradrenaline in cells isolated from the rabbit portal vein. The Journal of Physiology. 1988;404:557–573. doi: 10.1113/jphysiol.1988.sp017306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397:255–258. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- Colquhoun D. Practical analysis of single channel records. In: Standen NB, Gray PTA, Whitaker MJ, editors. Microelectrode Techniques. Cambridge: The Company of Biologists Limited; 1987. pp. 83–104. [Google Scholar]
- Colquhoun D, Hawkes AG. The principles of the stochastic interpretation of ion channel mechanisms. In: Sakmann B, Neher E, editors. Single Channel Recording. New York: Plenum Press; 1995. pp. 397–482. [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harteneck C, Plant TD, Schultz G. From worm to man: three subfamilies of TRP channels. Trends in Neurosciences. 2000;23:159–166. doi: 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- Helliwell RM, Large WA. Dual effect of external Ca2+ on nordrenaline-activated cation current in rabbit portal vein smooth muscle cells. The Journal of Physiology. 1996;492:75–88. doi: 10.1113/jphysiol.1996.sp021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell RM, Large WA. α-Adrenoceptor activation of a non-selective cation current in rabbit portal vein by 1,2-diacyl-sn-glycerol. The Journal of Physiology. 1997;499:417–428. doi: 10.1113/jphysiol.1997.sp021938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell RM, Large WA. Facilitatory effect of Ca2+ on the noradrenaline-evoked cation current in rabbit portal vein smooth muscle cells. The Journal of Physiology. 1998;512:731–741. doi: 10.1111/j.1469-7793.1998.731bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schltz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Inoue R, Kuriyama H. Dual regulation of cation-selective channels by muscarinic and α1-adrenergic receptors in the rabbit portal vein. The Journal of Physiology. 1993;465:427–448. doi: 10.1113/jphysiol.1993.sp019685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby KL, Pallotta BS. Burst kinetics of single calcium-activated potassium channels in cultured rat muscle. The Journal of Physiology. 1983;344:605–623. doi: 10.1113/jphysiol.1983.sp014958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Inoue R, Yamasaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imo K, Mori Y. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Journal of Biological Chemistry. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- Reuss H, Mojet MH, Chyb S, Hardie RC. In vivo analysis of the Drosophila light-sensitive channels. Neuron. 1997;13:837–848. doi: 10.1016/s0896-6273(00)80416-x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Large WA. Noradrenaline-evoked cation conductance recorded with the nystatin whole-cell method in rabbit portal vein cells. The Journal of Physiology. 1991;435:21–39. doi: 10.1113/jphysiol.1991.sp018496. [DOI] [PMC free article] [PubMed] [Google Scholar]


