Abstract
Parameters derived from frequency-domain analysis of heart period and blood pressure variability are gaining increasing importance in clinical practice. However, the underlying physiological mechanisms in human subjects are not fully understood. Here we address the question as to whether the low frequency variability (∼0.1 Hz) of the heart period may depend on a baroreflex-mediated response to blood pressure oscillations, induced by the α-sympathetic drive on the peripheral resistance.
Heart period (ECG), finger arterial pressure (Finapres) and respiratory airflow were recorded in eight healthy volunteers in the supine position with metronome respiration at 0.25 Hz. We inhibited the vascular response to the sympathetic vasomotor activity with a peripheral α-blocker (urapidil) and maintained mean blood pressure at control levels with angiotensin II.
We performed spectral and cross-spectral analysis of heart period (RR) and systolic pressure to quantify the power of low- and high-frequency oscillations, phase shift, coherence and transfer function gain.
In control conditions, spectral analysis yielded typical results. In the low-frequency range, cross-spectral analysis showed high coherence (> 0.5) and a negative phase shift (−65.1 ± 18 deg) between RR and systolic pressure, which indicates a 1–2 s lag in heart period changes in relation to pressure. In the high-frequency region, the phase shift was close to zero, indicating simultaneous fluctuations of RR and systolic pressure. During urapidil + angiotensin II infusion the low-frequency oscillations of both blood pressure and heart period were abolished in five cases. In the remaining three cases they were substantially reduced and lost their typical cross-spectral characteristics.
We conclude that in supine rest conditions, the oscillation of RR at low frequency is almost entirely accounted for by a baroreflex mechanism, since it is not produced in the absence of a 0.1 Hz pressure oscillation.
The results provide physiological support for the use of non-invasive estimates of the closed-loop baroreflex gain from cross-spectral analysis of blood pressure and heart period variability in the 0.1 Hz range.
Spontaneous oscillations of heart period and arterial pressure can be analysed by mathematical tools to extract the main frequency-related information. This is generally contained within three principal bands: a high-frequency (HF) band, related to the respiratory rate, a low-frequency (LF) band, centred around 0.1 Hz and a very-low-frequency (VLF) band, spanning the leftmost part of the spectrum.
Pressure oscillations at the respiratory frequency are linked to rhythmic changes of intrathoracic pressure due to respiratory mechanics, while low-frequency fluctuations, often identified as Mayer's waves, are the result of efferent sympathetic activity (Polosa, 1984; Cevese et al. 1995). The interpretation of heart period fluctuations is more problematic, because it has to account for the interplay of sympathetic and vagal outflows to the heart. The respiratory sinus arrhythmia (HF) certainly depends on vagal outflow (Katona & Jih, 1975) with possible sympathetic modulation (Hedman et al. 1995), while involvement of the baroreceptor control (Piepoli et al. 1997) is controversial (Cooke et al. 1999). The origin of LF oscillations of the heart period is even more uncertain, because different studies essentially based on similar approaches have led to contradictory interpretations, variously attributing a greater role either to sympathetic control (Weise et al. 1987; Guzzetti et al. 1994; Pagani et al. 1997), or to vagal outflow (Pomeranz et al. 1985; Koh et al. 1994; Jokkel et al. 1995; Grasso et al. 1997).
Concerning the origin of these LF waves, several investigations, on the basis of evidence for LF activity in vagal, as well as in sympathetic efferent fibres (Pagani et al. 1997), prompted the idea that a brainstem neural oscillator (Montano et al. 1996) independently generates the LF activity. Cooley et al. (1998) recently reported LF oscillations of heart period in two heart failure patients during blood pressure buffering, thus confirming this hypothesis. In contrast, several models have also been proposed to explain the LF oscillation of both heart period and blood pressure in terms of purely reflex mechanisms (de Boer et al. 1987; Madwed et al. 1989; Bernardi et al. 1994).
While the debate about the primary source of these waves is still open, a number of investigators have postulated that baroreflex control may be the main cause of heart rate fluctuations (de Boer et al. 1987; Grasso et al. 1997; Cooke et al. 1999) in response to blood pressure fluctuations generated by an oscillation of the sympathetic vasomotor tone. On this basis, it was suggested that spontaneous fluctuations should be used to investigate reflex cardiovascular control in closed-loop conditions. The LF and HF components were considered together to give a lumped baroreflex sensitivity estimate (Lucini et al. 1994). A trivariate model has also been recently introduced to estimate baroreflex and non-baroreflex control gains (Lucini et al. 2000).
Numerous papers are now available which validate the clinical use of parameters derived from frequency-domain analysis of heart period and arterial pressure variability in order to obtain an index of (closed-loop) baroreflex sensitivity (Pitzalis et al. 1998; James et al. 1998; Rudas et al. 1999). This has proved to be of diagnostic and prognostic value for a variety of cardiovascular diseases (Landolina et al. 1997; La Rovere et al. 1998). The underlying assumption, however, has yet to be explicitly demonstrated, though recent experiments on cats may provide support for it (Mancia et al. 1999). This method therefore cannot replace standard invasive tests, such as those based on pharmacological pressure manipulations. In any event, it is worth mentioning that the spectral estimates of baroreflex sensitivity correlate significantly, though loosely, with the estimates obtained using the phenylephrine method (Colombo et al. 1999).
In the present study in human subjects, we assessed the role of baroreflex control in determining the LF oscillation of heart period; we inhibited the sympathetic-induced oscillations of vasomotor tone with α-blockade, while maintaining mean blood pressure and mean heart rate close to control levels with angiotensin II. If LF oscillations of heart period were mainly caused by a direct β-sympathetic influence to the cardiac pacemaker, the effect of α-blockade should not lead to any substantial changes of these oscillations. On the other hand, if LF oscillations were due to transmission of LF pressure variability to the heart through the baroreflex, these oscillations should decrease both in heart period and blood pressure.
METHODS
This investigation conformed to the principles outlined in the Declaration of Helsinki and was approved by the local ethics committee. The experimental subjects were healthy volunteers (6 males and 2 females) aged 23-36 years and were taking no medications. They gave their written informed consent. We performed all the experiments in a protected environment, in an intensive care unit, in order to be in a position to handle any possible emergency related to the administration of a hypotensive drug.
Data collection
We recorded the ECG with a standard apparatus, blood pressure with a photoplethysmograph (Finapres, Ohmeda 2300, Englewood CO, USA), and respiratory airflow with a turbine-based spirometer connected to a mouthpiece. All signals were recorded on magnetic tape (TEACR71, Tokyo, Japan) and fed offline to an MS-DOS computer at a sampling rate of 1000 Hz with 12-bit A/D conversion.
Signal processing
We performed offline beat-to-beat analysis of the stored signals and obtained time series of successive values of heart period (RR), systolic (SP), diastolic (DP) and mean (MP) arterial pressures and instantaneous respiratory airflow (RESP). We checked the time series for ectopic beats, and substituted their values by linear interpolation of adjacent beats; in addition, we removed significant trends by subtracting the best-fitting regression line from the time series. Analysis was performed on 400-600 cardiac cycles taken from 10 min recordings. To perform the spectral analysis, we fitted an autoregressive monovariate model to each time series (Bartoli et al. 1985) and automatically quantified the power and central frequency associated with each spectral peak by computing the residuals (Johnsen & Andersen, 1978). We also performed cross-spectral analysis by fitting a bivariate autoregressive model to the RR and SP time series, to quantify the frequency-related squared coherence, the phase shift and the transfer function gain. The method is based on the same assumptions that underlie monovariate autoregressive modelling: each time series entering the estimation algorithm is the result of the addition of random noise and a weighted sum of previous values in both time series. In addition, the multichannel autoregressive process models reciprocal influences of the first variable on the second one and vice versa by summing weighted past values from the alternate time series. It is particularly suitable for the analysis of closed-loop interactions between linear systems under stationary conditions (Kubota et al. 1991; Patton et al. 1996; Nakata et al. 1998). The squared coherence, transfer function gain and phase shift between RR and SP were calculated after Fourier transformation of the multivariate autoregressive coefficients. The autoregressive coherence corresponds to the best estimate of the proportion of non-random variance common to both variables at a given frequency. We selected and recorded discrete values of phase shift and transfer function gain (TFG) between RR and SP in the low (TFG LF) and in the high (TFG HF) frequency regions at the frequencies corresponding to the coherence peak values, where the estimated error is minimal (Kay, 1991). These frequencies generally matched the central frequency of HF and LF peaks estimated for heart period and blood pressure by autoregressive spectral analysis. If, however, the central frequencies did not match exactly, the cross-spectral maximal coherence occurred at an intermediate frequency. Since this method provides a smooth estimate of the true cross-spectra, distinct values of transfer function gain and phase shift are smoothed estimates of the relation between the time series in the vicinity of the considered frequency. TFG LF quantifies the slope of the linear relationship between RR and SP (units are ms mmHg−1) provided the coherence is higher than the conventionally accepted limit of 0.5 (Pagani et al. 1986; Mancia et al. 1999). Because of the identical units, TFG has been used as an estimate of baroreflex sensitivity (Robbe et al. 1987).
Experimental protocol
Experiments were performed early in the afternoon. Subjects were allowed to take a light breakfast at least 4 h prior to the experiment. They were fully instrumented for recording ECG, blood pressure and respiratory flow. We compared the readings of the pressure recording device with the sphygmomanometry readings, accepting a maximum difference of ± 10 mmHg, and changed the position of the finger cuff until this criterion was fulfilled. We inserted an i.v. cannula with a 3-way infusion port in the antecubital vein and immediately started a slow saline drip to maintain patency. The other two injection lines were connected to infusion pumps (Beckton & Dickinson, Franklin Lakes, NJ, USA) for simultaneous controlled administration of two substances. Subjects lay quietly in the supine position, until the absence of evident heart rate and mean blood pressure trends demonstrated that good baseline conditions had been achieved. We invited all subjects to synchronise their respiratory movements with the acoustic pace of a metronome beating at 0.25 Hz, while headphones diffused soft music. We controlled the respiratory rate to avoid overlapping between low frequency oscillations and respiratory arrhythmia, which may spontaneously occur in supine resting (Cooke et al. 1998). It has been reported that synchronised respiration does not alter the prevailing vagal tone in this condition (Patwardhan et al. 1995). We recorded the signals for 10 min in the supine resting condition. We then injected urapidil (Uraprene, IBI, Milan, Italy) in successive boluses of 50 mg, to obtain α-adrenergic blockade (van Zwieten & Chalmers, 1994). In preliminary observations we had found that each bolus produced a transient decrease of arterial pressure, while several repetitions of bolus injections induced a steady effect. Thus, we gave further boluses at 1 min intervals until mean arterial pressure remained steadily reduced, by at least 20 %. We then kept up this steady α-blockade by constant perfusion of urapidil 0.15 mg kg−1 min−1. Once this situation was established, we raised blood pressure towards control values by infusing angiotensin II (INALCO, Milan, Italy) in successive boluses of 500 ng followed by perfusion of 6 ng kg−1 min−1. We recorded the signals for another 10 min period during steady-state perfusion of urapidil and angiotensin II (treatment). At the end of the experiments, we withheld urapidil, while continuing the perfusion of angiotensin II and gradually reducing it, until the hypotensive effect of α-blockade disappeared. All subjects experienced some degree of dizziness, nausea and weakness, and were kept in bed under observation for at least 1 h after the end of drug administration. These symptoms, however, appeared late after the end of the recording sessions, and were unrelated to the actual level of blood pressure. The subjects received cumulative doses of urapidil and angiotensin II of 250-350 mg and 8-9 μg, respectively.
Statistical analysis
Data are expressed as means ±s.d. We tested the significance of differences between data recorded in control periods and during α-blockade and angiotensin II infusion by Student's t test for paired data. When appropriate, logarithmic transformation of data was also performed, to reduce skewness; the results of such transformation, however, did not influence the outcome of the statistical tests.
RESULTS
An example of spectral and cross-spectral analysis from a representative subject in the control condition is shown in Fig. 1. The top three diagrams show power spectral densities (units2 Hz−1) from the RR, SP and RESP beat-to-beat time series. Shaded areas show how the powers (units2) related to the LF and HF bands were estimated. Vertical lines indicate the estimated central frequencies of LF and HF. The next three diagrams show the outcome of cross-spectral analysis from the same time series. Phase spectra are in degrees, squared coherences are pure numbers and TFGs are expressed in units of ms mmHg−1, consistent with units of the baroreflex gain, as computed by other methods (Mary & Hainsworth, 1993). Coherence and TFG spectra present the highest values at LF (0.12 Hz) and HF (0.25 Hz), where SP and RR fluctuations are closely correlated. Mean LF TFG (11.3 in Fig. 1) across all subjects in control conditions was 9.7 ± 5.0 ms mmHg−1, which corresponds to average values reported in other studies using similar methods. As already reported by others (de Boer et al. 1987; Cooke et al. 1999), the phase shift at LF lies between −60 and −90 deg (-80 deg in Fig. 1), which corresponds to a delay of RR relative to SP of 1–2 heart beats, while at HF it tends to zero, indicating that RR and SP respiration-related oscillations occur simultaneously. The experiments included three steps: control, α-blockade alone and α-blockade plus angiotensin II infusion (treatment). Spectral analysis was systematically performed, however, in control conditions and during full treatment. As an example, we report, in Fig. 2, 1 min sections of tracings recorded in one subject in the three conditions. In this experiment, α-blockade reduced SP from 138 to 105 mmHg and RR from 957 to 822 ms. Angiotensin II restored SP to 136 mmHg and RR to 959 ms. Respiration-related oscillations of both RR and SP were preserved in all conditions.
Figure 1. Example of spectral and cross-spectral analysis in control conditions.
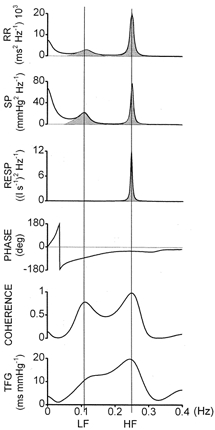
From top to bottom: power spectra of heart period (RR), systolic pressure (SP) and respiratory airflow (RESP); phase, coherence and transfer function gain (TFG) between RR and SP. Stippled areas show how powers were calculated; vertical lines identify points of maximal coherence in low (LF) and high (HF) frequency ranges, for reading exact frequency, TFG and phase shift (see text for further details).
Figure 2. Example of recordings in the three sets of conditions.
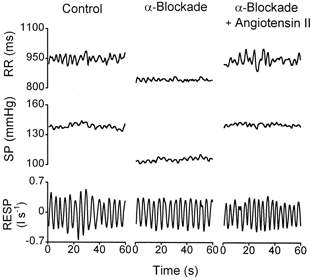
One minute time series of heart period (RR), systolic pressure (SP) and respiratory airflow (RESP) in one subject in control conditions (left), during α-blockade alone (centre) and during α-blockade plus angiotensin II (right). α-Blockade reduced average SP and RR, which were restored to control values after infusion of angiotensin II. Respiration was not altered in any of the conditions and respiration-related oscillations of RR and SP were always clearly visible.
Time-domain data
Average results (mean ±s.d.) for heart period (RR), systolic (SP), diastolic (DP) and mean (MP) blood pressure are shown in Table 1. RR, DP and MP were not statistically different before and after treatment, while SP decreased by 10 %. The treatment also reduced the total variance of all the variables, but statistical significance was reached for heart period only.
Table 1. Time-domain results.
| RR(ms) | RR variance (ms2) | SP(mmHg) | SP variance(mmHg2) | DP(mmHg) | DP variance(mmHg2) | MP(mmHg) | MP variance(mmHg2) | |
|---|---|---|---|---|---|---|---|---|
| Control | 768 ± 85 | 1331 ± 380 | 142 ± 11 | 26.6 ± 21.1 | 78 ± 9 | 7.7 ± 2.5 | 100 ± 12 | 11.3 ± 3.9 |
| Treatment | 712 ± 95 | 841 ± 567 | 126 ± 10 | 12.0 ± 10.4 | 73 ± 8 | 5.7 ± 5.7 | 93 ± 11 | 7.7 ± 7.6 |
| P | 0.16 | 0.047 | 0.0001 | 0.18 | 0.1 | 0.23 | 0.15 | 0.34 |
Mean values ± s.d. of heart period (RR), systolic (SP), diastolic (DP) and mean (MP) arterial pressure, and their respective variance in 8 subjects, before (Control) and during administration of urapidil and angiotensin II (Treatment). P, statistical significance of changes between control and treatment by paired t test.
Spectral analysis
All the subjects displayed the usual LF (0.1 ± 0.01 Hz) and HF (0.25 ± 0.01) peaks in control conditions (Fig. 1). The mean values for control and treatment are reported in Table 2. In contrast to the central frequency, all power values showed a fairly high degree of dispersion, which is consistent with that described in previous studies (Montano et al. 1994). In Fig. 3 we report the LF power values from individual experiments. The treatment induced dramatic changes in low frequency oscillations, with a systematic drop in LF powers. In five cases (subjects 1, 3, 4, 5 and 6), both RR and SP oscillations became almost undetectable in the frequency region close to 0.1 Hz. In the remaining three cases, LF oscillations were still visible, but with very low powers: in subject 2, LF SP changed from 1.95 to 0.34 mmHg2 and LF RR from 148 to 61 ms2; in subject 7, LF SP changed from 2.26 to 0.43 mmHg2 and LF RR from 170 to 32 ms2; in subject 8, LF SP changed from 6.43 to 0.64 mmHg2 and LF RR from 528 to 36 ms2.
Table 2. Frequency-domain results: spectral analysis.
| Control | Treatment | |
|---|---|---|
| RR | ||
| LF power (ms2) | 363 ± 139 | 20 ± 21*** |
| HF power (ms2) | 266 ± 274 | 119 ± 212** |
| SP | ||
| LF power (mmHg2) | 3.2 ± 1.8 | 0.2 ± 0.2*** |
| HF power (mmHg2) | 1.5 ± 0.8 | 1.3 ± 0.9 |
| MP | ||
| LF power (mmHg2) | 2.2 ± 1.0 | 0.2 ± 0.1*** |
| HF power (mmHg2) | 0.8 ± 0.4 | 0.7 ± 0.7 |
| DP | ||
| LF power (mmHg2) | 2.0 ± 1.0 | 0.2 ± 0.1*** |
| HF power (mmHg2) | 0.4 ± 0.4 | 0.4 ± 0.3 |
| RESP | ||
| HF power ((l s-1)2) | 4.4 ± 4.7 | 2.6 ± 1.6 |
Mean values ± s.d. of powers of heart period (RR), systolic pressure (SP), mean pressure (MP), diastolic pressure (DP) and respiratory airflow (RESP), in low (LF) and high (HF) frequency range in 8 subjects, before (Control) and during administration of urapidil and angiotensin II (Treatment).
P < 0.01;
P < 0.001.
Figure 3. Low frequency powers of heart period and systolic pressure.
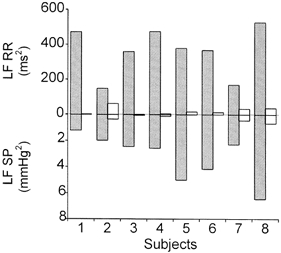
Individual values of LF powers of heart period (RR, upward) and systolic pressure (SP, downward) in 8 subjects in control conditions (stippled bars) and during α-blockade plus angiotensin II (open bars). During the treatment LF waves were practically undetectable in 5 subjects, in the remaining 3 they were strongly reduced.
The respiration-related oscillations conserved the same frequency before and during the treatment. This indicates that the subjects were able to pace their respiratory movements according to the metronome in all steps of the experiments. In addition, respiratory mechanics were little altered, as suggested by the lack of significant changes in airflow power (Table 2). The HF power of SP also remained unchanged, suggesting that the respiratory oscillations of arterial pressure are related to a non-sympathetic mechanism. In contrast, the HF power of RR showed a significant 55 % decrease (P < 0.01).
Cross-spectral analysis
An example of spectral and cross-spectral analysis before and during the treatment from subject 6 is shown in Fig. 4. In this case, LF (∼0.1 Hz) and HF (∼0.25 Hz) peaks are clearly detected in the control condition both for RR and SP (continuous lines). During α-blockade plus angiotensin II, the LF peaks are practically invisible, whereas a pronounced new peak appears centred around 0.04 Hz. The RR HF peak is also reduced, as described above, though it is still related to respiratory activity. The cross-spectral parameters indicate the typical results obtained in control conditions in healthy subjects, characterised by high coherence between RR and SP in both the LF and HF regions, a negative phase shift in LF, and a near-zero degrees phase shift in HF; TFG LF was about 8 ms mmHg−1 (Grasso et al. 1997). The negative phase shift in LF indicates that RR oscillations lag behind SP oscillations with a delay of 1–2 heart beats. The situation after treatment changed drastically, since around 0.1 Hz the coherence fell to low levels, while it rose to more than 0.5 at the new reduced-frequency peak. At 0.05 Hz, the phase shift wrapped around ± 180 deg, indicating a complete phase opposition between RR and SP (see also Fig. 5). At the respiratory frequency, coherence and phase shifts were not significantly changed, confirming that the mechanism responsible for HF RR variability was almost unaffected by the experimental manoeuvre.
Figure 4. Example of spectral and cross-spectral analysis in control conditions and during the treatment.
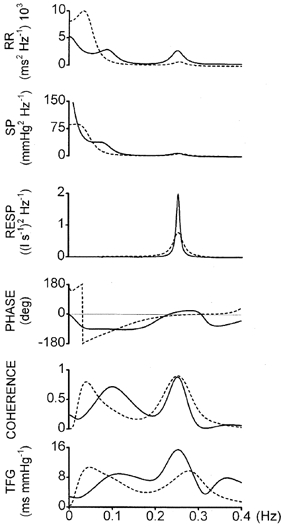
Spectral and cross-spectral analysis of time series of heart period (RR), systolic pressure (SP) and respiratory airflow (RESP), from one subject in control conditions (continuous line) and during α-blockade plus angiotensin II (dashed line). Phase shift, PHASE, squared coherence, COHERENCE, transfer function gain between RR and SP, TFG. Average time-domain values in control and during the treatment: RR = 847 and 785 ms; SP = 149 and 131 mmHg. In control, high coherence oscillations at LF (0.1 Hz), with negative phase shift, and at HF (0.25 Hz), with near-zero phase shift, can be seen. During the treatment, HF oscillations have lower power and TFG, but similar phase relationship and coherence, while the LF component is abolished (low coherence at 0.1 Hz); new reduced-frequency (0.04 Hz) oscillations, in complete phase opposition, appear in RR and SP.
Figure 5. The ‘reduced low frequency’.
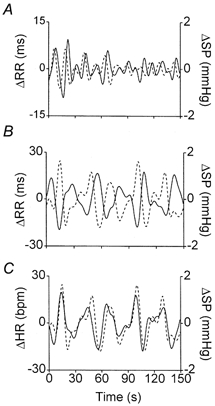
Same subject as in Fig. 4. Two strips of the original time series of RR (continuous line, left axis) and SP (dashed line, right axis) were differentiated and low-pass filtered (cutoff at 0.15 Hz), to boost oscillations at frequencies below 0.15 Hz. A, control conditions; B, during α-blockade plus angiotensin II; C, same as B, but RR converted to its reciprocal (i.e. heart rate, HR). In A the frequency of oscillations is ∼0.1 Hz (LF); all peaks and valleys of SP lead those of RR; in B the frequency of oscillations is ∼0.05 Hz (rLF); peaks and valleys approximately oppose each other; in C, peaks and valleys almost coincide, indicating that HR and SP change in the same direction, with no phase shift.
The mean values of cross-spectral analysis from the whole group of subjects are reported in Table 3. The cross-spectral characteristics in the LF range in control conditions were in agreement with those reported in previous studies in healthy subjects in similar experimental conditions (Baselli et al. 1986; Grasso et al. 1997). The average values of cross-spectral analysis in the LF range after treatment are not reported in Table 3, because in five cases low-frequency oscillations of both SP and RR were not detected. In two of the remaining cases (subjects 7 and 8) coherence fell to low values, while in the last case (subject 2) the phase shift became more negative (from -63.43 to -108.6 deg, coherence 0.61). Thus, in these three cases, the residual low-frequency oscillations after treatment seem to depend on different mechanisms, as compared with the physiological condition.
Table 3. Frequency-domain results: cross-spectral analysis.
| LF Control (8) | HF Control (8) | HF Treatment (8) | rLF Treatment (5) | |
|---|---|---|---|---|
| Frequency (Hz) | 0.1 ± 0.01 | 0.25 ± 0.01 | 0.25 ± 0.01 | 0.044 ± 0.012 |
| Phase (deg) | −65.1 ± 18.2 | −28.5 ± 29.9 | −50.5 ± 44.7 | −125.2 ± 39.7 |
| Coherence | 0.76 ± 0.15 | 0.85 ± 0.11 | 0.82 ± 0.19 | 0.64 ± 0.15 |
| TFG (ms mmHg-1) | 9.18 ± 4.48 | 9.69 ± 5.0 | 6.5 ± 4.8* | 6.26 ± 4.6 |
Mean values ± s.d. of frequency, phase, coherence and transfer function gain (TFG) between RR and SP, in low (LF), high (HF) and reduced-low-frequency (rLF) range. Control, before administration of urapidil and angiotensin II; Treatment, during administration of urapidil and angiotensin II. Number of subjects indicated in parentheses. LF waves were not seen during the treatment, and rLF waves were seen only during the treatment (see text).
P < 0.05.
In the HF range, coherence and phase shift were unchanged after treatment. On the other hand, TFG HF showed a relative, but significant, decrease from 9.7 ± 5.0 to 6.5 ± 4.8 ms mmHg−1 (P < 0.05).
Reduced low-frequency waves
In five subjects (three of whom showed complete abolition of 0.1 Hz oscillations), we observed well defined lower-frequency waves, whose spectral and cross-spectral characteristics are also reported in Table 3. The spectral powers of RR and SP were 731 ms2 (range 69-2178 ms2) and 17.4 mmHg2 (range 0.7-50 mmHg2), respectively, the frequency was centred around 0.05 (a 20 s period), the coherence was well over 0.5 and the average phase shift was −125 deg (range −73 to −169 deg). The frequency of these oscillations is in the lower part of, or just below, the range generally classified as LF, but not as low as what is usually indicated as VLF (Task Force European Society of Cardiology and North American Society of Pacing and Electrophysiology, 1996). We have therefore adopted a new acronym (rLF = reduced-low-frequency) as applying specifically to these waves, which were by no means new in our experience, as will be discussed below. What is typical of rLF is that the phase shift between RR and SP becomes more negative, tending towards phase opposition (Fig. 4), which means that the reciprocal of RR (i.e. heart rate, HR) fluctuates simultaneously and in phase with SP. In order to assess the phase relationship between the two variables (Cevese et al. 1995), in Fig. 5 we plotted the differentiated low-pass filtered (cutoff at 0.15 Hz) SP and HR time series. This procedure does not change the phase relationship between the two variables. HR and SP are closely related and, in contrast with our findings in the LF range in control conditions, the HR waves now coincide with or slightly precede the SP fluctuations. This was confirmed in all five subjects.
DISCUSSION
The principal interpretation of the present results is that the low-frequency oscillation of the RR interval is induced by a sympathetically mediated oscillation of total peripheral resistance (Montano et al. 1996) that acts as a stimulus for the baroreceptors. The prerequisite for this putative role of the baroreceptors is the phase shift between the RR and SP time series, which normally indicates a lag of heart rate fluctuations in relation to SP, corresponding, in the time domain, to 1–2 cardiac cycles. If a different phase shift is encountered (such as 0 deg, or 180 deg), a mechanism other than the cardiovascular reflexes must be postulated.
In a previous study (Grasso et al. 1997), we suggested that a parasympathetic mechanism involving the baroreflex might be responsible for LF fluctuations at the sinus node. We had found that RR interval fluctuations at 0.1 Hz in human subjects were neither abolished nor significantly reduced after β-blockade, in agreement with observations by others (Pomeranz et al. 1985; Jokkel et al. 1995). The phase shift and coherence between RR and SP remained unaltered compared with control conditions. We therefore suggested that a low-frequency oscillatory pressure input, generated by a sympathetically mediated oscillation of total peripheral resistance (Montano et al. 1996) may act as a stimulus for the baroreceptors. We tested this hypothesis here by selectively disrupting that part of the efferent branch of the sympathetic system which has been shown to provide a rhythmic vasomotor drive leading to blood pressure oscillations (Polosa, 1984; Cevese et al. 1995). We found that in five out of eight subjects the treatment abolished the LF oscillations of SP and the LF oscillations of heart period as well. In the remaining three subjects, the treatment drastically reduced LF SP power, but was unable to completely abolish the LF pressure oscillation; this may reasonably be attributed to less complete α-blockade. In these three cases, LF RR fluctuations mimicked the drop in LF SP and lost coherence or the typical phase relationship.
Why did LF RR interval fluctuations disappear after α-blockade but not after β-blockade? If the sympathetic vasomotor tone oscillates at 0.1 Hz, inducing LF SP waves, then the efferent sympathetic drive to the heart should also oscillate with a similar frequency. We suggest, however, that, in physiological conditions, the effect of such oscillation will normally be obscured by the much stronger baroreceptor-mediated vagal influences, since the vago-sympathetic interference (Levy & Martin, 1979) leads to vagal dominance in the control of heart performance. Reflex vagal fluctuations affect the heart with a time lag which is compatible with the delay in the baroreflex arc (Borst & Karemaker, 1983; Mary & Hainsworth, 1993). On the other hand, the effects of the efferent sympathetic oscillation on the heart should be unmasked by α-blockade. We propose that these effects are found in rLF, as explained here below.
Reduced LF
In five cases we detected fairly stable, well organised rLF oscillations. Since in the present study, we were not specifically addressing these oscillations, we cannot provide a complete description and a sound explanation of this phenomenon. Nevertheless, we decided to include this finding in the Results and Discussion, because we believe that rLF should not be regarded simply as slowed LF. In rLF the phase shift between RR and SP oscillations tended towards complete phase opposition. As shown in Fig. 5, this means that heart rate accelerates or decelerates in accordance with the raising or lowering of SP. This is opposite to what one would expect as the result of the baroreflex mechanism. We therefore suggest that in this case oscillations of SP may be the direct consequence of slow changes in heart rate (Ferrari et al. 1996), because α-adrenergic effects on total peripheral resistance were abolished and β-adrenergic effects (if any) should lead to reciprocal changes. We found a similar phase opposition between RR and SP oscillations at reduced frequency in a previous study on anaesthetised dogs with or without carotid sinus buffering, as well as before and after vagotomy (Cevese et al. 1995; Grasso et al. 1995). Thus, in special conditions, when the baroreceptor activity is impaired, as in the case of anaesthetised dogs, or the sympathetically driven vasomotor input at 0.1 Hz is blunted, as after α-blockade, a direct sympathetic oscillatory drive to the heart may prevail (Polosa, 1984; Montano et al. 1996; Lambertz & Langhorst, 1998).
We can only speculate as to the significance of the reduced low frequency. One possibility is that the delay in the transmission of neurochemical signals from sympathetic endings to sinoatrial effector cells may filter off an oscillation at 0.1 Hz, leading to a half-frequency oscillation of RR (Yoshida et al. 1994). Another possibility is that incoming signals from the baroreceptors interfere with spontaneously oscillating centres at the brainstem level, altering their natural frequency (Lambertz & Langhorst, 1998). It is worthy of note that, in a pathological condition in which the baroreceptor activity is reduced and the control of the peripheral resistance impaired, as in pre-syncope, a shift of LF to lower-frequency values has also been observed (Lipsitz et al. 1997).
Cooley et al. (1998) reported slow oscillations of heart period in the absence of oscillations of arterial pressure, and suggested a central sympathetic origin of heart period oscillations. Their study, however, does not report numerical values of frequency. Close inspection of the figures (see Fig. 1 and 2 of their paper) suggests that the frequency may well be about 0.05 Hz. This may account for the discrepancy between their interpretation of LF and ours, if the LF oscillations they reported are identified with the rLF we have described here.
Limitations
The recovery of arterial pressure and heart rate with angiotensin II was essentially satisfactory. The residual small reduction in SP may be accounted for by incomplete compensation of the effect of α-blockade on the capacitance vessels by angiotensin, slightly limiting venous return and stroke volume. We did not completely restore SP, since this would have caused an increase in MP beyond the control levels.
We also observed a significant reduction in RR total variance and in the power of HF RR, though the coherence and phase shift of the relationship between RR and SP at HF never showed significant changes. It has been demonstrated that angiotensin II inhibits central vagal tone (Townend et al. 1995) and enhances sympathetic outflow (Matsukawa et al. 1991), while resetting the baroreceptors to higher pressure levels (Mace et al. 1985; Townend, 1996). It might therefore be suggested that the substantial abolition of LF RR in the present experiments was also simply due to the central effects of angiotensin II. During angiotensin II infusion, however, we regularly noticed a progressive slowing down of heart rate towards control values, concomitant with arterial pressure restoration (see Fig. 2). If the baroreceptors were reset, vagal tone impaired and sympathetic outflow enhanced, all our subjects should have been tachycardic when normal blood pressure was restored after α-blockade. To reconcile this observation with the central effects of angiotensin, it should be noted that the amount of the peptide we infused was in the lower range of the amounts reported by others and that we were operating in the leftmost part of the baroreceptor function curve. In other words, we were raising a lowered arterial pressure, not elevating it above control levels. In conclusion, though angiotensin II may have been responsible for reduced modulation of vagal activity, probably contributing to a reduction of the overall variance of RR (Vaile et al. 1998), there were no signs of compromised baroreflex function.
Conclusions
Since, as previously discussed, the dominant mechanism of 0.1 Hz RR interval fluctuations is the baroreceptor-mediated vagal influence, our findings provide strong physiological support for the use of TFG LF as an index of baroreflex sensitivity in human subjects. Conversely, using TFG HF for this purpose would appear to be of limited value.
It should, however, be recalled that the model we propose applies to, and has been tested in, resting supine subjects presenting no pathological condition. On the other hand, the appearance of lower-frequency oscillations (rLF) with different cross-spectral features indicates that in special cases the control system may modify its characteristics.
Acknowledgments
The authors wish to thank Miss Giuliana Cerutti for her invaluable help with data analysis and the preparation of the manuscript and Mr Gaetano Guglielmi for his assistance in the Intensive Care Unit.
Renato Grasso died on October 6, 2000. We, the authors, wish to dedicate this paper to Renato, whose contribution to the development and realisation of the experiments, as well as the writing and reviewing of the text, was essential. His brilliant intellectual and scientific mind, as well as his warm friendship, will remain forever in our hearts.
References
- Bartoli F, Baselli G, Cerutti S. AR identification and spectral estimate applied to the R-R interval measurements. International Journal of Biomedical Computing. 1985;16:201–215. doi: 10.1016/0020-7101(85)90055-8. [DOI] [PubMed] [Google Scholar]
- Baselli G, Cerutti S, Civardi S, Liberati D, Lombardi F, Malliani A, Pagani M. Spectral and cross-spectral analysis of heart rate and arterial blood pressure variability signals. Computers and Biomedical Research. 1986;19:520–534. doi: 10.1016/0010-4809(86)90026-1. [DOI] [PubMed] [Google Scholar]
- Bernardi L, Leuzzi S, Radaelli A, Passino C, Johnston JA, Sleight P. Low-frequency spontaneous fluctuations of R-R interval and blood pressure in conscious humans: a baroreceptor or central phenomenon? Clinical Science. 1994;87:649–654. doi: 10.1042/cs0870649. [DOI] [PubMed] [Google Scholar]
- Borst C, Karemaker JM. Time delays in the human baroreceptor reflex. Journal of the Autonomic Nervous System. 1983;9:399–409. doi: 10.1016/0165-1838(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Cevese A, Grasso R, Poltronieri R, Schena F. Vascular resistance and arterial pressure low-frequency oscillations in the anaesthetized dog. American Journal of Physiology. 1995;268:H7–16. doi: 10.1152/ajpheart.1995.268.1.H7. [DOI] [PubMed] [Google Scholar]
- Colombo R, Mazzuero G, Spinatonda G, Lanfranchi P, Giannuzzi P, Ponikowki P, Coats AJS, Minuco G. Comparison between spectral analysis and the phenylephrine method for the assessment of baroreflex sensitivity in chronic heart failure. Clinical Science. 1999;97:503–513. [PubMed] [Google Scholar]
- Cooke WH, Cox JF, Diedrich AM, Taylor JA, Beightol LA, Ames JEI, Hoag JB, Seidel H, Eckberg DL. Controlled breathing protocols probe human autonomic cardiovascular rhythms. American Journal of Physiology. 1998;274:709–H718. doi: 10.1152/ajpheart.1998.274.2.h709. [DOI] [PubMed] [Google Scholar]
- Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KUO, Eckberg DL. Human responses to upright tilt: a window on central autonomic integration. Journal of Physiology. 1999;517:617–628. doi: 10.1111/j.1469-7793.1999.0617t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley RL, Montano N, Cogliati C, Van De Borne P, Richenbacher W, Oren R, Somers VK. Evidence for a central origin of the low-frequency oscillation in RR-interval variability. Circulation. 1998;98:556–561. doi: 10.1161/01.cir.98.6.556. [DOI] [PubMed] [Google Scholar]
- De Boer RW, Karemaker JM, Strackee J. Hemodynamic fluctuations and baroreflex sensitivity in humans: a beat-to-beat model. American Journal of Physiology. 1987;253:H680–689. doi: 10.1152/ajpheart.1987.253.3.H680. [DOI] [PubMed] [Google Scholar]
- Ferrari AU, Franzelli C, Daffonchio A, Perlini S, Dirienzo M. Sympathovagal interplay in the control of overall blood pressure variability in unanesthetized rats. American Journal of Physiology. 1996;270:H2143–2148. doi: 10.1152/ajpheart.1996.270.6.H2143. [DOI] [PubMed] [Google Scholar]
- Grasso R, Rizzi G, Schena F, Cevese A. Arterial baroreceptors are not essential for low frequency oscillation of arterial pressure. Journal of the Autonomic Nervous System. 1995;50:323–331. doi: 10.1016/0165-1838(94)00103-q. [DOI] [PubMed] [Google Scholar]
- Grasso R, Schena F, Gulli G, Cevese A. Does low-frequency variability of heart period reflect a specific parasympathetic mechanism? Journal of the Autonomic Nervous System. 1997;63:30–38. doi: 10.1016/s0165-1838(96)00128-2. [DOI] [PubMed] [Google Scholar]
- Guzzetti S, Cogliati C, Broggi C, Carozzi C, Caldiroli D, Lombardi F, Malliani A. Influences of neural mechanisms on heart period and arterial pressure variabilities in quadriplegic patients. American Journal of Physiology. 1994;266:H1112–1120. doi: 10.1152/ajpheart.1994.266.3.H1112. [DOI] [PubMed] [Google Scholar]
- Hedman AE, Tahvanainen KUO, Hartikainen JEK, Hakumaki MOK. Effect of sympathetic modulation and sympatho-vagal interaction on heart rate variability in anaesthetized dogs. Acta Physiologica Scandinavica. 1995;155:205–214. doi: 10.1111/j.1748-1716.1995.tb09965.x. [DOI] [PubMed] [Google Scholar]
- James MA, Panerai RB, Potter JF. Applicability of new techniques in the assessment of arterial baroreflex sensitivity in the elderly: a comparison with established pharmacological methods. Clinical Science. 1998;94:245–253. doi: 10.1042/cs0940245. [DOI] [PubMed] [Google Scholar]
- Johnsen SJ, Andersen N. On power estimation in maximum entropy spectral analysis. Geophysics. 1978;43:681–690. [Google Scholar]
- Jokkel G, Bonyhay I, Kollai M. Heart rate variability after complete autonomic blockade in man. Journal of the Autonomic Nervous System. 1995;51:85–89. doi: 10.1016/0165-1838(95)80010-8. [DOI] [PubMed] [Google Scholar]
- Katona PG, Jih F. Respiratory sinus arrhythmia: noninvasive measure of parasympathetic cardiac control. Journal of Applied Physiology. 1975;39:801–805. doi: 10.1152/jappl.1975.39.5.801. [DOI] [PubMed] [Google Scholar]
- Kay SM. Modern Spectral Estimation: Theory and Application. Englewood Cliffs, New Jersey: Prentice-Hall Inc; 1991. [Google Scholar]
- Koh J, Brown TE, Beightol LA, Ha CY, Eckberg DL. Human autonomic rhythms: vagal cardiac mechanisms in tetraplegic subjects. Journal of Physiology. 1994;474:483–495. doi: 10.1113/jphysiol.1994.sp020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Itaya R, Alexander J, Jr, Todaka K, Sugimachi M, Sunagawa K. Autoregressive analysis of aortic input impedance: comparison with Fourier transform. American Journal of Physiology. 1991;260:H998–1002. doi: 10.1152/ajpheart.1991.260.3.H998. [DOI] [PubMed] [Google Scholar]
- Lambertz M, Langhorst P. Simultaneous changes of rhythmic organization in brainstem neurons, respiration, cardiovascular system and EEG between 0. 05 Hz and 0.5 Hz. Journal of the Autonomic Nervous System. 1998;68:58–77. doi: 10.1016/s0165-1838(97)00126-4. [DOI] [PubMed] [Google Scholar]
- Landolina M, Mantica M, Pessano P, Manfredini R, Foresti A, Schwartz PJ, De Ferrari GM. Impaired baroreflex sensitivity is correlated with hemodynamic deterioration of sustained ventricular tachycardia. Journal of American College of Cardiology. 1997;29:568–575. doi: 10.1016/s0735-1097(96)00533-5. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Bigger JTJ, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (autonomic tone and reflexes after myocardial infarction) investigators [see comments] Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- Levy MN, Martin PJ. Neural control of the heart. In: Berne RM, Sperelakis N, Geiger SR, editors. Handbook of Physiology, The Heart. I. Bethesda, MD, USA: American Physiolological Society; 1979. pp. 581–600. section 2. [Google Scholar]
- Lipsitz LA, Morin R, Gagnon M, Kiely D, Medina A. Vasomotor instability preceding tilt-induced syncope: does respiration play a role? Journal of Applied Physiology. 1997;83:383–390. doi: 10.1152/jappl.1997.83.2.383. [DOI] [PubMed] [Google Scholar]
- Lucini D, Pagani M, Mela GS, Malliani A. Sympathetic restraint of baroreflex control of heart period in normotensive and hypertensive subjects. Clinical Science. 1994;86:547–556. doi: 10.1042/cs0860547. [DOI] [PubMed] [Google Scholar]
- Lucini D, Porta A, Milani O, Baselli G, Pagani M. Assessment of arterial and cardiopulmonary baroreflex gains from simultaneous recordings of spontaneous cardiovascular and respiratory variability. Journal of Hypertension. 2000;18:281–286. doi: 10.1097/00004872-200018030-00007. [DOI] [PubMed] [Google Scholar]
- Mace PJE, Watson RDS, Skan W, Littler WA. Inhibition of the baroreceptor heart rate reflex by angiotensin II in normal man. Cardiovascular Research. 1985;19:525–527. doi: 10.1093/cvr/19.9.525. [DOI] [PubMed] [Google Scholar]
- Madwed JB, Albrecht P, Mark RG, Cohen RJ. Low-frequency oscillations in arterial pressure and heart rate: a simple computer model. American Journal of Physiology. 1989;256:H1573–1579. doi: 10.1152/ajpheart.1989.256.6.H1573. [DOI] [PubMed] [Google Scholar]
- Mancia G, Parati G, Castiglioni P, Di Rienzo M. Effect of sinoaortic denervation of frequency-domain estimates of baroreflex sensitivity in conscious cats. American Journal of Physiology. 1999;276:H1987–1993. doi: 10.1152/ajpheart.1999.276.6.H1987. [DOI] [PubMed] [Google Scholar]
- Mary DASG, Hainsworth R. Methods for the study of cardiovascular reflexes. In: Hainsworth R, Mark AL, editors. Cardiovascular Reflex Control in Health and Disease. London: Saunders W.B. Co.; 1993. pp. 1–34. [Google Scholar]
- Matsukawa T, Gotoh E, Minamisawa K, Kihara M, Ueda S-I, Shionoiri H, Ishii M. Effects of intravenous infusions of angiotensin II on muscle sympathetic nerve activity in humans. American Journal of Physiology. 1991;261:R690–696. doi: 10.1152/ajpregu.1991.261.3.R690. [DOI] [PubMed] [Google Scholar]
- Montano N, Gnecchi-Ruscone T, Porta A, Lombardi F, Malliani A, Barman SM. Presence of vasomotor and respiratory rhythms in the discharge of single medullary neurons involved in the regulation of cardiovascular system. Journal of the Autonomic Nervous System. 1996;57:116–122. doi: 10.1016/0165-1838(95)00113-1. [DOI] [PubMed] [Google Scholar]
- Montano N, Gnecchi-Ruscone T, Porta A, Lombardi F, Pagani M, Malliani A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation. 1994;90:1826–1831. doi: 10.1161/01.cir.90.4.1826. [DOI] [PubMed] [Google Scholar]
- Nakata A, Takata S, Yuasa T, Shimakura A, Maruyama M, Nagai H, Sakagami S, Kobayashi K-I. Spectral analysis of heart rate, arterial pressure, and muscle sympathetic nerve activity in normal humans. American Journal of Physiology. 1998;274:H1211–1217. doi: 10.1152/ajpheart.1998.274.4.H1211. [DOI] [PubMed] [Google Scholar]
- Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell'Orto S, Piccaluga E, Turiel M, Baselli G, Cerutti S, Malliani A. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circulation Research. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Pagani M, Montano N, Porta A, Malliani A, Abboud F, Birkett C, Somers VK. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation. 1997;95:1441–1448. doi: 10.1161/01.cir.95.6.1441. [DOI] [PubMed] [Google Scholar]
- Patton DJ, Triedman JK, Perrott MH, Vidian AA, Saul JP. Baroreflex gain: characterization using autoregressive moving average analysis. American Journal of Physiology. 1996;270:H1240–1249. doi: 10.1152/ajpheart.1996.270.4.H1240. [DOI] [PubMed] [Google Scholar]
- Patwardhan AR, Evans JM, Bruce EN, Eckberg DL, Knapp CF. Voluntary control of breathing does not alter vagal modulation of heart rate. Journal of Applied Physiology. 1995;78:2087–2094. doi: 10.1152/jappl.1995.78.6.2087. [DOI] [PubMed] [Google Scholar]
- Piepoli M, Sleight P, Leuzzi S, Valle F, Spadacini G, Passino C, Johnston J, Bernardi L. Origin of respiratory sinus arrhythmia in conscious humans. Circulation. 1997;95:1813–1821. doi: 10.1161/01.cir.95.7.1813. [DOI] [PubMed] [Google Scholar]
- Pitzalis MV, Mastropasqua F, Passantino A, Massari F, Ligurgo L, Forleo C, Balducci C, Lombardi F, Rizzon P. Comparison between noninvasive indices of baroreceptor sensitivity and the phenylephrine method in post-myocardial infarction patients. Circulation. 1998;97:1362–1367. doi: 10.1161/01.cir.97.14.1362. [DOI] [PubMed] [Google Scholar]
- Polosa C. Rhythms in the activity of the autonomic nervous system: their role in the generation of systemic arterial pressure waves. In: Miyakawa K, Koepchen HP, Polosa C, editors. Mechanisms of Blood Pressure Waves. Tokyo: Japanese Science Society Press; 1984. pp. 27–41. [Google Scholar]
- Pomeranz B, Macaulay RJB, Caudill MA, Kutz I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Cohen RJ, Benson H. Assessment of autonomic function in humans by heart rate spectral analysis. American Journal of Physiology. 1985;248:H151–153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- Robbe HWJ, Mulder LJM, Ruddel H, Langewitz WA, Veldman JBP, Mulder G. Assessment of baroreceptor reflex sensitivity by means of spectral analysis. Hypertension. 1987;15:538–543. doi: 10.1161/01.hyp.10.5.538. [DOI] [PubMed] [Google Scholar]
- Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KUO, Kuusela TA, Eckberg DL. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. American Journal of Physiology. 1999;276:H1691–1698. doi: 10.1152/ajpheart.1999.276.5.h1691. [DOI] [PubMed] [Google Scholar]
- Task Force European Society Of Cardiology And North American Society Of Pacing And Electrophysiology. Heart rate variability. (Standards of measurement physiological interpretation and clinical use) Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Townend JN. Angiotensin II as a modulator of cardiovascular autonomic control. Cardiologia. 1996;41:217–225. [PubMed] [Google Scholar]
- Townend JN, Al-Ani M, West JN, Littler WA, Coote JH. Modulation of cardiac autonomic control in humans by angiotensin II. Hypertension. 1995;25:1270–1275. doi: 10.1161/01.hyp.25.6.1270. [DOI] [PubMed] [Google Scholar]
- Vaile JC, Fletcher J, Littler WA, Coote JH, Townend JN. Angiotensin II modulates cardiovascular autonomic control in the absence of baroreflex loading. Heart. 1998;80:127–133. doi: 10.1136/hrt.80.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zwieten PA, Chalmers JP. Different types of centrally acting antihypertensives and their targets in the central nervous system. Cardiovascular Drugs and Therapy. 1994;8:787–799. doi: 10.1007/BF00877397. [DOI] [PubMed] [Google Scholar]
- Weise F, Heydenreich F, Runge U. Contributions of sympathetic and vagal mechanisms to the genesis of heart rate fluctuations during orthostatic load: a spectral analysis. Journal of the Autonomic Nervous System. 1987;21:127–134. doi: 10.1016/0165-1838(87)90015-4. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Harasawa Y, Kubota T, Chishaki H, Kubo T, Sunagawa K, Takeshita A. Role of carotid sinus baroreflex in attenuating systemic arterial pressure variability studied in anesthetized dogs. American Journal of Physiology. 1994;266:H720–729. doi: 10.1152/ajpheart.1994.266.2.H720. [DOI] [PubMed] [Google Scholar]


