Abstract
The aim of this study was to describe the time course of the response of human muscle protein synthesis (MPS) to a square wave increase in availability of amino acids (AAs) in plasma. We investigated the responses of quadriceps MPS to a ≈1.7-fold increase in plasma AA concentrations using an intravenous infusion of 162 mg (kg body weight)−1 h−1 of mixed AAs. MPS was estimated from D3-leucine labelling in protein after a primed, constant intravenous infusion of D3-ketoisocaproate, increased appropriately during AA infusion.
Muscle was separated into myofibrillar, sarcoplasmic and mitochondrial fractions. MPS, both of mixed muscle and of fractions, was estimated during a basal period (2.5 h) and at 0.5-4 h intervals for 6 h of AA infusion.
Rates of mixed MPS were not significantly different from basal (0.076 ± 0.008 % h−1) in the first 0.5 h of AA infusion but then rose rapidly to a peak after 2 h of ≈2.8 times the basal value. Thereafter, rates declined rapidly to the basal value. All muscle fractions showed a similar pattern.
The results suggest that MPS responds rapidly to increased availability of AAs but is then inhibited, despite continued AA availability. These results suggest that the fed state accretion of muscle protein may be limited by a metabolic mechanism whenever the requirement for substrate for protein synthesis is exceeded.
It is now generally accepted that amino acid availability per se is an independent regulator of muscle protein turnover, with amino acids stimulating muscle protein synthesis (Bennet et al. 1989). Nevertheless, because the time resolution of the methods used for determination of protein synthesis in human muscle has been relatively poor, most investigations of the effects of amino acids (and of other anabolic stimuli) have hitherto used measurement periods of 3 h or more (Smith & Rennie, 1996; Ljungqvist et al. 1997). Nearly all studies (except those using the flooding dose technique (Smith & Rennie, 1996) show that feeding or administration of amino acids stimulates muscle protein synthesis about 1.5- to 3-fold. However, it is unlikely that provision of amino acids or proteins results in an indefinite stimulation of muscle growth, so either the process of synthesis must become limited despite continued availability of amino acids, or muscle protein breakdown must also be elevated. Our major aim was to test the first of these possibilities by describing the time course of the response to a square wave increase in the availability of amino acids. We also wished to test the possibility that the different major classes of muscle proteins, i.e. the myofibrillar, soluble and mitochondrial proteins, would show differential effects to the continued availability of amino acids.
METHODS
All subjects gave informed written consent before participating in the study, which was approved by the Institutional Review Board of the University of Texas Medical Branch at Galveston, TX. We studied six normal, healthy volunteers (5 men, 1 woman, 33 ± 1 years, 80 ± 5 kg (mean ±s.e.m.) in the post-absorptive state, before and during a primed (54 mg kg−1) constant infusion of mixed amino acids (162 mg kg−1 h−1, Table 1) as Aminosyn 15 % (Abbott, Deerfield, IL, USA). The subjects were admitted to the Clinical Research Centre of the University of Texas Medical Branch the evening before the study and ate nothing between 24.00 h and coming to the laboratory early in the morning of the study. Forearm veins were cannulated for the infusion of tracer amino acid and also for blood sampling. A primed (13.2 μmol kg−1) constant infusion of D3-α-ketoisocaproate (D3-KIC, 99 atoms %, 0.22 μmol kg−1 min−1, Cambridge Isotopes Inc., Cambridge, MA, USA), which is rapidly transaminated in muscle, was used to deliver tracer leucine to the muscle (Chinkes et al. 1996). The delivery of the D3-KIC was increased about 2.2-fold to match the increased availability of leucine given during the mixed amino acid infusion. Blood samples were taken before the tracer infusion and at 30-60 min for up to 9 h. Biopsies of vastus lateralis muscle were taken from both legs under local anaesthetic (lidocaine (1 %)) using Tilley-Henkel forceps (Helliwell et al. 1987) at 30 and 180 min of the basal period (before amino acid infusion) and then at 30-240 min intervals during 6 h of the amino acid infusion. One portion of each muscle biopsy (20-50 mg) was immediately frozen; a larger portion (100-120 mg) was immediately homogenized in a high-salt buffer containing protease and phosphatase inhibitors (20 mm Hepes, 2 mm EGTA, 50 mm NaF, 50 mm KCl, 0.2 mm EDTA, 50 mmβ-glycerophosphate, 0.1 μm PMSF, 1 mm benzamidine, 0.5 mm sodium orthovanadate, 1 μm microcystin LR and 1 mm dithiothreitol). The resulting homogenate was subjected to a scheme of differential centrifugation and further salt extraction in order to prepare fractions of myofibrillar, sarcoplasmic and mitochondrial proteins. Centrifugation for 15 min at 1500 g was used to precipitate the myofibrillar fraction. The supernatant, which contained mitochondria and sarcoplasmic proteins, was subjected to centrifugation at 7000 g for 15 min to precipitate the mitochondria. The remaining supernatant was mainly sarcoplasmic proteins. Myofibrillar precipitates were washed twice in low salt buffer (100 mm KCl, 5 mm Tris-HCl, 1 mm DTT, pH 7.4). Mitochondria were washed once in a high ionic strength buffer (100 mm KCl, 5 mm EDTA, 5 mm MgSO4, 1 mm ATP and 50 mm Tris-HCl; pH 7.4). The protein in the sarcoplasmic fraction was precipitated by bringing the solution to 70 % with ethanol and the precipitate collected by low speed centrifugation (700 g). Aliquots of the myofibrillar and mitochondrial fractions were fixed overnight in 2 % glutaraldehyde in 0.08 m sodium cacodylate buffer, pH 7.4, washed and post-fixed in cacodylate buffered 1 % osmium tetroxide. An equal volume of buffered 2 % glutaraldehyde was added to the sarcoplasmic supernatant fraction for fixing whereupon 100 μl of 3 % agar solution was added to 200 μl of the fixed fraction. Agar blocks were post-fixed and processed to plastic as described above. All fractions were then dehydrated and resin embedded. Ultrathin sections, 80 nm, from each sample were stained with lead citrate and uranyl acetate and photographed with a JEOL 100CX transmission electron microscope. Examination of aliquots showed a predominance of myofibrils and mitochondria in their respective fractions, with no crossover, and a lack of contamination of the sarcoplasmic fraction with mitochondria or myofibrils (results not shown).
Table 1.
Serum amino acid (AA) concentrations measured before and during the infusion of Aminosyn II 15%
| Baseline [AA] (μM) | Infusion rate (mmol kg−1 h−1) | [AA] during infusion (μM) | Fold increase | |
|---|---|---|---|---|
| Non-essential AAs | ||||
| Asp | 34 ± 3 | 0.085 | 83 ± 14 | 2.6 ± 0.4 |
| Glu | 135 ± 16 | 0.081 | 258 ± 45 | 2 ± 0.4 |
| Gly | 346 ± 40 | 0.108 | 647 ± 62 | 1.9 ± 0.2 |
| Ala | 464 ± 37 | 0.181 | 839 ± 83 | 1.8 ± 0.1 |
| Ser | 218 ± 14 | 0.082 | 368 ± 43 | 1.7 ± 0.1 |
| Gln | 784 ± 48 | — | 889 ± 66 | 1.1 ± 0.1 |
| Asn | 81 ± 3 | — | 57 ± 4 | 0.7 ± 0.1 |
| Tyr | 107 ± 14 | 0.024 | 78 ± 12 | 0.7 ± 0.1 |
| Essential AAs | ||||
| Arg | 115 ± 9 | 0.095 | 352 ± 41 | 3.1 ± 0.2 |
| Trp | 21 ± 2 | 0.016 | 56 ± 8 | 2.9 ± 0.8 |
| Ile | 73 ± 4 | 0.082 | 196 ± 15 | 2.7 ± 0.2 |
| Lys | 236 ± 30 | 0.116 | 591 ± 71 | 2.6 ± 0.1 |
| Leu | 177 ± 12 | 0.123 | 418 ± 26 | 2.4 ± 0.1 |
| Thr | 176 ± 6 | 0.054 | 299 ± 13 | 1.7 ± 0.1 |
| Met | 28 ± 2 | 0.019 | 47 ± 1 | 1.7 ± 0.1 |
| Val | 234 ± 20 | 0.069 | 348 ± 22 | 1.5 ± 0.1 |
| His | 125 ± 8 | 0.031 | 181 ± 14 | 1.4 ± 0.1 |
| Phe | 72 ± 3 | 0.029 | 96 ± 4 | 1.3 ± 0.1 |
Concentrations of each amino acid increased significantly P < 0.01 during the amino acid infusion (except for Asn and Tyr, which decreased). Values are means ± S.E.M.
Unfortunately due to a technical failure we lost so many samples from the mitochondrial fractions that only two complete sets were available. However we have reported these since they were internally consistent in the changes observed in each set of seven samples.
Protein fractions were dissolved in 0.5 m NaOH then hydrolysed for 24 h with 6 m HCl at 100 °C. Plasma was precipitated using 15 % sulphosalicylic acid. The amino acids from the HCl hydrolysate and in the sulphosalicylic supernatant were separated using strong cation exchange chromatography, derivatized using n-acetyl N-propyl reagents and subjected to selective ion monitoring gas chromatography mass spectrometry using a Hewlett Packard 5890 GCMS in the electron impact mode (Chinkes et al. 1996). We monitored the m+3/m+2 ratio of the D3-containing adduct. The standard curve of detector response vs. enrichment was linear from 0 to 0.2 % tracer/tracee for D3-leucine, with our samples mostly falling in the range 0.05-0.1 %. The rate of muscle protein synthesis was calculated by a comparison of the extent of labelling in the protein fractions with the labelling of the D3-leucine in blood. Previous work has shown that there is no significant difference in the extent of labelling between free intramuscular leucine labelling and that of arterial or venous blood (Chinkes et al. 1996). The rates were expressed as per cent per hour (% h−1). Serum amino acid concentrations were determined by HPLC. Serum glucose was determined using the glucose oxidase reaction with a Sigma kit (Sigma Diagnostics, St Louis, MO, USA); serum insulin was determined using a radioimmunoassay (Coat-A-Count kit, Diagnostic Products Corporation, Los Angeles, CA, USA). Serum urea was measured using a colorimetric method with coupled enzyme reactions involving urease and glutamate dehydrogenase (Sigma Diagnostics).
Statistical analysis
Data are expressed as means ±s.e.m.n = 6. The mean values for rates of synthesis were compared using analysis of variance with repeated measures. The same analysis was performed for serum amino acid concentrations (Table 1). The null hypothesis was rejected at the 5 % level.
RESULTS
Amino acids and urea
Mean plasma amino acid concentrations rose significantly P < 0.01≈1.7-fold from 3.4 ± 0.2 mm as a result of the amino acid infusion (Table 1). There was no change in the concentration of most amino acids during the 6 h of infusion, but tyrosine and asparagine concentrations fell progressively by ≈50 % of their initial starting values. Asparagine was not present in the amino acid infusion mixture, and its tyrosine concentration was low (4.05 mg ml−1). Plasma urea was stable in the basal period at 3.4 ± 0.6 mm and changed little in the first 2.5 h of the amino acid infusion but then rose progressively to 5.9 ± 0.5 mm after 6 h (P < 0.05 vs. 3 h period before amino acid infusion). The enrichment of D3-leucine in blood rapidly (within 30 min of infusion) achieved a plateau value of 7.2 ± 0.2 % atoms % excess. Although there was a slight (10 %) fall in D3-leucine enrichment during the period of amino acid infusion, despite the compensatory increase in D3-KIC infusion, this was not statistically significant (data not shown).
Blood glucose and serum insulin
Basal blood glucose was 70 ± 4 mg (100 ml)−1 and rose to 83 ± 7 mg (100 ml)−1 immediately after amino acid infusion, thereafter remaining steady (Fig. 1). Serum insulin concentration rose by ≈3-fold within the first 30 min of amino acid infusion and then fell over the next 2.5 h of amino acid infusion to a value slightly higher than that in the basal state, but not significantly different from it.
Figure 1. Time course of serum insulin and glucose.
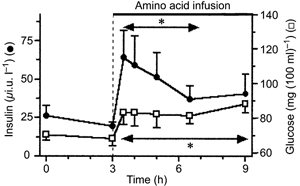
*Values for insulin and glucose significantly above basal values.
Muscle protein synthesis
The rate of incorporation of labelled leucine into mixed muscle protein was 0.08 ± 0.01 % h−1 in the basal state. There was no detectable change within the first 30 min of amino acid infusion but thereafter the rate of incorporation rose rapidly and significantly P < 0.05 (to 0.21 ± 0.07 between 30 and 60 min and 0.24 ± 0.04 % h−1 between 60 and 120 min of amino acid infusion) (Fig. 2). Between 120 and 360 min of amino acid infusion the rate of mixed muscle synthesis fell markedly from the peak value, becoming not significantly different from the basal value (Fig. 2). The responses in the myofibrillar, sarcoplasmic and mitochondrial fractions (Fig. 3) were very similar in pattern and relative extent to that observed in the mixed muscle protein.
Figure 2. Time course of rate of synthesis of mixed muscle proteins.
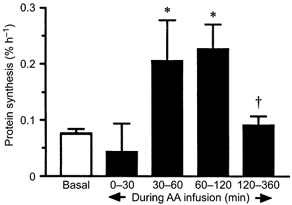
*P < 0.05 vs. basal; †P < 0.05 vs. peak value.
Figure 3. Rates of synthesis of mixed muscle proteins and muscle fractions (myofibrillar, sarcoplasmic and mitochondrial proteins) during the basal period and during the infusion of the amino acid solution.
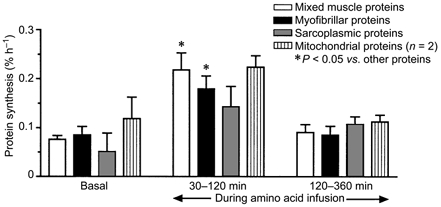
The values shown are the averages over the time periods shown, estimated from biopsies taken at the beginnings and ends of the periods.
DISCUSSION
The major findings of this study are (a) that a square-wave increase in the availability of plasma amino acids took between 30 min and 1 h to have any measurable effect on muscle protein synthesis, whereupon (b) MPS was markedly stimulated by ≈2.8-fold for a period of 1.5 h before falling to a value not significantly different from the basal value for the succeeding 4 h. Furthermore, the responses of protein synthesis in the myofibrillar, sarcoplasmic and mitochondrial fractions show essentially the same pattern as shown in the mixed muscle proteins.
Novel features of these results are the description of the length of the latent period before stimulation of muscle protein synthesis by amino acids and the observation of the reduction in the synthetic rate to basal values, neither of which was previously known. The existence of the reduction makes sense, both in terms of our previous knowledge and teleologically. Overfeeding protein does not increase the size of the lean body mass, and amino acids supplied in excess of the requirements of protein synthesis are simply oxidized (Motil et al. 1981; Price et al. 1994) and their carbon skeletons used for fuel or stored as fat. The rise in plasma urea observed in this study supports this interpretation.
The mechanism of the fall in muscle protein synthesis is unknown. Our data showed that there was a marked rise in the availability of plasma insulin during the early part of the amino acid infusion but that plasma insulin concentration fell thereafter. The rise and fall of plasma insulin are somewhat similar in their extent and duration to the rise and fall of the rate of muscle protein synthesis, consistent with there being a causal link. The question of whether or not insulin stimulates muscle protein synthesis remains somewhat controversial with not all authors agreeing (Gelfand & Barrett, 1986; Bennet et al. 1990; Biolo et al. 1995; Biolo et al. 1999). However, in our view, although insulin may increase muscle protein synthesis in the presence of a sufficiency of amino acids this effect is mainly permissive with the major effect of insulin being to decrease muscle protein breakdown. In more recent, preliminary studies (J. Bohé, J. F. A. Low, R. R. Wolfe and M. J. Rennie, unpublished observations) in which we infused amino acids at three different rates between 43 and 261 mg kg−1 h−1, we observed increases in muscle synthesis of the same order as seen here with a 3-fold range of insulin values, supporting the view that insulin is mainly permissive above a certain relatively low value. In our opinion, the major anabolic drive occurring when amino acid availability is increased is the amino acid concentration per se, and this is supported by the findings that insulin plays little part in the meal-induced increase in muscle synthesis seen in rats (Yoshizawa et al. 1997, 1998). However, this interpretation requires that some signalling pathways initially stimulated by increased amino acid availability are later turned off or inhibited. The most likely candidates include the eukaryotic initiation factors, which have been shown to be activated by increasing the availability of amino acids to rat muscle both in vivo and in vitro (Yoshizawa et al. 1998; Vary et al. 1999). It may be that there is some feedback mechanism, which effectively inhibits the amino acid stimulation of the initiation of protein synthesis during prolonged amino acid availability. This remains to be tested.
The final possibility is that the decreased availability of asparagine and tyrosine limited muscle protein synthesis in our subjects. This remains a possibility but, in our view, a remote one. Neither asparagine not tyrosine is a major component of muscle protein and the continued high availability of amino acids to which they are metabolically related (e.g. aspartate, glutamine and phenylalanine) suggests that there was not a limitation of supply.
The finding that all fractions of the muscle protein mass showed the same pattern of response suggests that the control mechanisms for regulating the rate of protein synthesis with respect to amino acid availability are likely to be common and not organelle specific as might have been conceived for the mitochondria, for example.
The present results suggest that during a normal day, the extent of fed state accretion of muscle protein may become limited by an inhibitory mechanism (as yet unknown) whenever the substrate requirement for protein synthesis is exceeded. The system may act like a protein-stat, reacting to a signal from the ‘bag-full’ state of muscle, as conceptualized by Millward (1995).
One major lesson to be learned from the present results is that studies of the effects of possible modulators of human muscle protein metabolism need to be made with greater time resolution than previously. The findings also have substantial implications for the use of amino acids in total parenteral nutrition and also for the concept of protein requirements. It may be that amino acids are more efficiently utilized for maintaining lean body mass and providing substrate for wound healing when given in divided doses (as occurs with meal feeding) rather than with continuous application. Furthermore the oversupply of amino acids beyond the currently identified requirement when given continuously may have no benefit in stimulating tissue synthesis but merely stimulate the induction of enzymes of amino acid catabolism (Price et al. 1994).
Acknowledgments
This work was supported by Shriners Hospital Grant 8490 and NIH Grants M01-00073 and DK 38010. We thank Dr Dennis Gore for clinical coverage and Dr Judah Rosenblatt for statistical analysis and Robert Cox and Dr Hal Hawkins for the electron microscopy. M.J.R. thanks the University of Dundee for sabbatical leave and the UK Medical Research Council for support. J.B. thanks the Hospices Civils de Lyon and the Ville de Lyon (Prix Innovation 1998) for financial support.
References
- Bennet WM, Connacher AA, Scrimgeour CM, Jung RT, Rennie MJ. Euglycemic hyperinsulinemia augments amino acid uptake by human leg tissues during hyperaminoacidemia. American Journal of Physiology. 1990;259:E185–194. doi: 10.1152/ajpendo.1990.259.2.E185. [DOI] [PubMed] [Google Scholar]
- Bennet WM, Connacher AA, Scrimgeour CM, Smith K, Rennie MJ. Increase in anterior tibialis muscle protein synthesis in healthy man during mixed amino acid infusion: studies of incorporation of [1-13C]leucine. Clinical Science. 1989;76:447–454. doi: 10.1042/cs0760447. [DOI] [PubMed] [Google Scholar]
- Biolo G, Fleming RY D, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. Journal of Clinical Investigation. 1995;95:811–819. doi: 10.1172/JCI117731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biolo G, Williams BD, Fleming RYD, Wolfe RR. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes. 1999;48:949–957. doi: 10.2337/diabetes.48.5.949. [DOI] [PubMed] [Google Scholar]
- Chinkes D, Klein S, Zhang XJ, Wolfe RR. Infusion of labeled KIC is more accurate than labeled leucine to determine human muscle protein synthesis. American Journal of Physiology. 1996;270:E67–71. doi: 10.1152/ajpendo.1996.270.1.E67. [DOI] [PubMed] [Google Scholar]
- Gelfand RA, Barrett EJ. Effect of physiological hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. Journal of Clinical Investigation. 1986;80:1–6. doi: 10.1172/JCI113033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell TR, Coakley J, Smith PE, Edwards RH. The morphology and morphometry of the normal human tibialis anterior muscle. Neuropathology and Applied Neurobiology. 1987;13:297–307. doi: 10.1111/j.1365-2990.1987.tb00069.x. [DOI] [PubMed] [Google Scholar]
- Ljungqvist OH, Persson M, Ford GC, Nair KS. Functional heterogeneity of leucine pools in human skeletal muscle. American Journal of Physiology. 1997;36:E564–570. doi: 10.1152/ajpendo.1997.273.3.E564. [DOI] [PubMed] [Google Scholar]
- Millward DJ. A protein-stat mechanism for the regulation of growth and maintenance of the lean-body mass. Nutrition Research Reviews. 1995;8:93–120. doi: 10.1079/NRR19950008. [DOI] [PubMed] [Google Scholar]
- Motil KJ, Matthews DE, Bier DM, Burke JF, Munro HN, Young VR. Whole-body leucine and lysine metabolism: response to dietary protein intake in young men. American Journal of Physiology. 1981;240:E712–721. doi: 10.1152/ajpendo.1981.240.6.E712. [DOI] [PubMed] [Google Scholar]
- Price GM, Halliday D, Pacy DJ, Quevedo RM, Millward DJ. Nitrogen homeostasis in man: 1. Influence of protein intake on the amplitude of diurnal cycling of body nitrogen. Clinical Science. 1994;86:91–102. doi: 10.1042/cs0860091. [DOI] [PubMed] [Google Scholar]
- Smith K, Rennie MJ. The measurement of tissue protein turnover. Bailliére's Clinical Endocrinology and Metabolism. 1996;10:469–495. doi: 10.1016/s0950-351x(96)80651-3. [DOI] [PubMed] [Google Scholar]
- Vary TC, Jefferson LS, Kimball SR. Amino acid-induced stimulation of translation initiation in rat skeletal muscle. American Journal of Physiology. 1999;277:E1077–1086. doi: 10.1152/ajpendo.1999.277.6.E1077. [DOI] [PubMed] [Google Scholar]
- Yoshizawa F, Kimball SR, Jefferson LS. Modulation of translation initiation in rat skeletal muscle and liver in response to food intake. Biochemical and Biophysical Research Communications. 1997;240:825–831. doi: 10.1006/bbrc.1997.7652. [DOI] [PubMed] [Google Scholar]
- Yoshizawa F, Kimball SR, Vary TC, Jefferson LS. Effect of dietary protein on translation initiation in rat skeletal muscle and liver. American Journal of Physiology. 1998;275:E814–820. doi: 10.1152/ajpendo.1998.275.5.E814. [DOI] [PubMed] [Google Scholar]


