Figure 1. Extracellular ATP (1 mm) generates two whole-cell currents with different time courses: an inward current (due to the activation of a non-selective cation channel) and an outward current (due to the activation of a K+-selective channel).
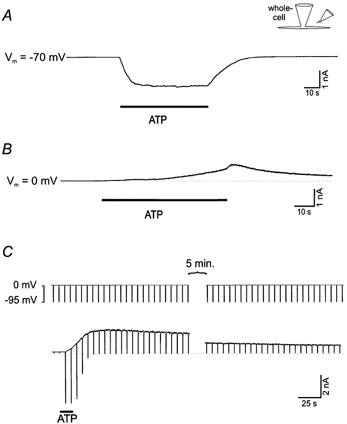
A, extracellular ATP activates a P2Z-like non-selective cation channel, which is best seen at negative holding potentials as an inward current. B, extracellular ATP also activates a delayed current, which is best seen as an outward current when the cell membrane potential is held at 0 mV (near the reversal potential for the P2Z-like channel current). C, the cell membrane potential was held at 0 mV to record the outward current and briefly (for 0.5 s every 6 s) changed to −95 mV (the calculated reversal or equilibrium potential for the K+ current) to record the non-selective cation current. The inward cation current depends on the continued presence of ATP while the outward current at 0 mV lasts for minutes after ATP application is terminated. Solutions A and C were used for these experiments. The reason for the brief additional increase of the outward current in B (sometimes) seen when the ATP application ceased is not clear. It may be partially due to some or all of the following: the series resistance, an increase in the extracellular Ca2+ concentration (see Fig. 4) as ATP (which chelates Ca2+, Table 2) is washed away, or an effect of cessation of pressure ejection. The stimulus protocol used for C was also used for Figs 3B, 4 and 5.
