Figure 3. Phosphorylation of kNBC1 by PKA.
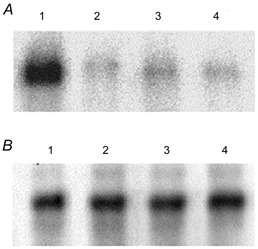
A, in vitro phosphorylation of WT kNBC1 (lanes 1 and 2) and the S982A-kNBC1 mutant (lanes 3 and 4) isolated from untreated mPCT cells (lanes 1 and 3) or cells treated with 100 μm 8-Br-cAMP (lanes 2 and 4) for 15 min. Immunoprecipitated EGFP-WT-kNBC1 and the EGFP-S982A-kNBC1 mutant were eluted from protein A-Sepharose beads and then phosphorylated in vitro using the PKA catalytic subunit and [γ-32P]ATP. A representative experiment is shown. B, the amount of the wild-type and mutant kNBC1 proteins used in these in vitro phosphorylation experiments was quantified using Western blotting with an affinity purified rabbit polyclonal kNBC1 antibody (Bok et al. 2001). Treatment of the cells with 8-Br-cAMP did not affect the size of EGFP-kNBC1 and the EGFP-S982A-kNBC1 mutant.
