Fig. 6. 3-D cryo-EM reconstructions of ΔN-Vps4p, full-length Vps4p and Vta1p-Vps4p complexes.
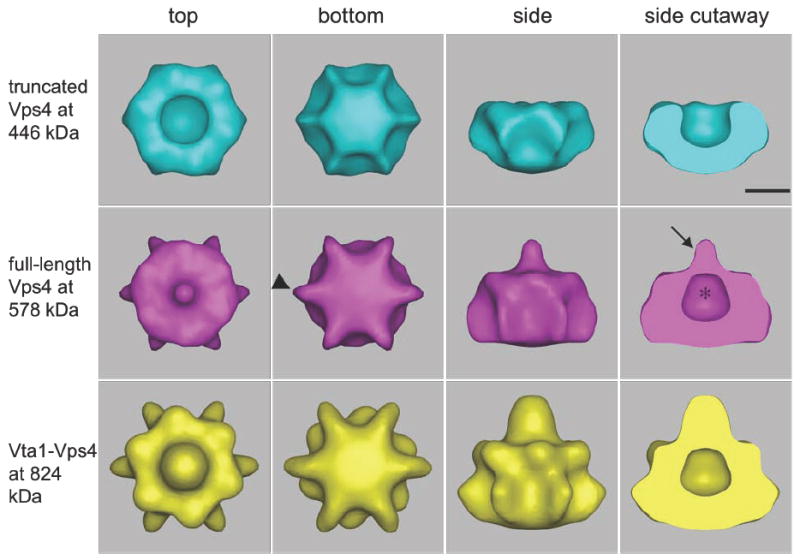
Isosurfaces are shown, contoured to enclose the full expected molecular weights of the ΔN-Vps4p, full-length Vps4p, and Vta1p-Vps4p complexes. All three complexes form bowl-like structures with a central cavity (asterisk) in the upper half, suggesting that the top and bottom rings are in very different conformations. The presence of the N-terminal MIT domain in full-length Vps4p causes a nipple (arrow) to appear above the cavity as well as six small fin-like densities (arrowheads) around the lower ring. The binding of Vta1p produces additional density mainly over and around the nipple and beneath the bottom ring. Scale bar 50 Å.
