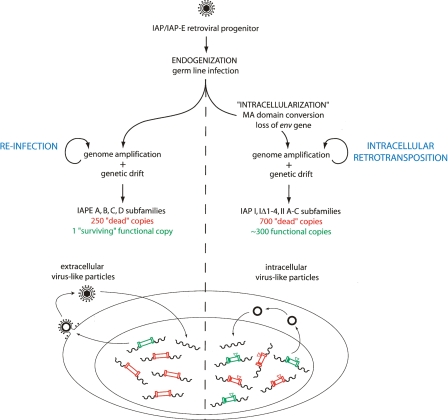
Figure 7.
A model for the “intracellularization” of infectious retroviruses. A bona fide infectious retrovirus—for example, the IAP/IAPE progenitor—is “endogenized” upon infection of the germline of a remote ancestor and Mendelian transmission to the following generations, thus resulting in a so-called Endogenous RetroVirus (ERV) (de Parseval and Heidmann 2005; Bannert and Kurth 2006). This ERV may retain the characteristics of retroviruses, that is, produce infectious extracellular particles with a functional envelope protein, which are prone to horizontal transmission and can also amplify in their proper host genome by reinfection of the germline (the IAPEs). “Intracellularization” is expected to correspond to a further adaptation, in which the produced virus-like particles are no more able to exit or to re-enter the cell. For IAPs this has been achieved via the conversion of the plasma membrane targeting function of the Gag MA domain with the acquisition of a signal for Gag-targeting to the ER membrane, and the loss of the env gene. The resulting strictly intracellular life cycle resembles that of primitive Ty-like LTR-retrotransposons (although both classes of elements should be evolutionarily distinct) and is associated—at least in the case of the IAP element—with high-efficiency retrotransposition. For both types of elements, that is, the infectious IAPEs and the strictly intracellular IAPs, defective elements resulting from genetic drift (e.g., point mutation, deletion, recombination, reverse transcription error, DNA-editing) can be complemented in trans by active copies, resulting in the present-day multiple-copy IAP and IAPE subfamilies, with a single “surviving” functional IAPE element, but ∼300 full-length autonomous IAP copies still present due to their high “multicopying” activity.