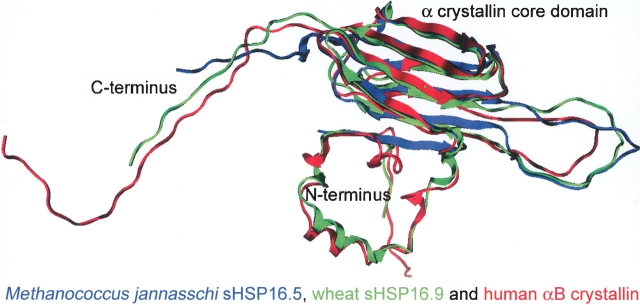
Figure 4.
Superposition of the monomeric subunits of Mj sHSP16.5 (blue), wheat sHSP16.9 (green), and human αB crystallin (red). The 3D coordinates of the three structures were superimposed yielding a final RMSD of 3.25 Å. All three structures have the same basic topology. The hydrophobic N terminus is largely helical (except in Mj sHSP16.5, where the N terminus is unresolved in the X-ray structure). An immunoglobulin-like α crystallin core domain consists of β strands (11 in Mj sHSP16.5, eight in wheat sHSP16.9, and seven in αB crystallin) that form two anti-parallel β sheets connected by a flexible loop. The C-terminal extension is highly charged and unstructured. Note that the C-terminal extension is unresolved in the X-ray structure of Mj sHSP16.5.