Figure 6.
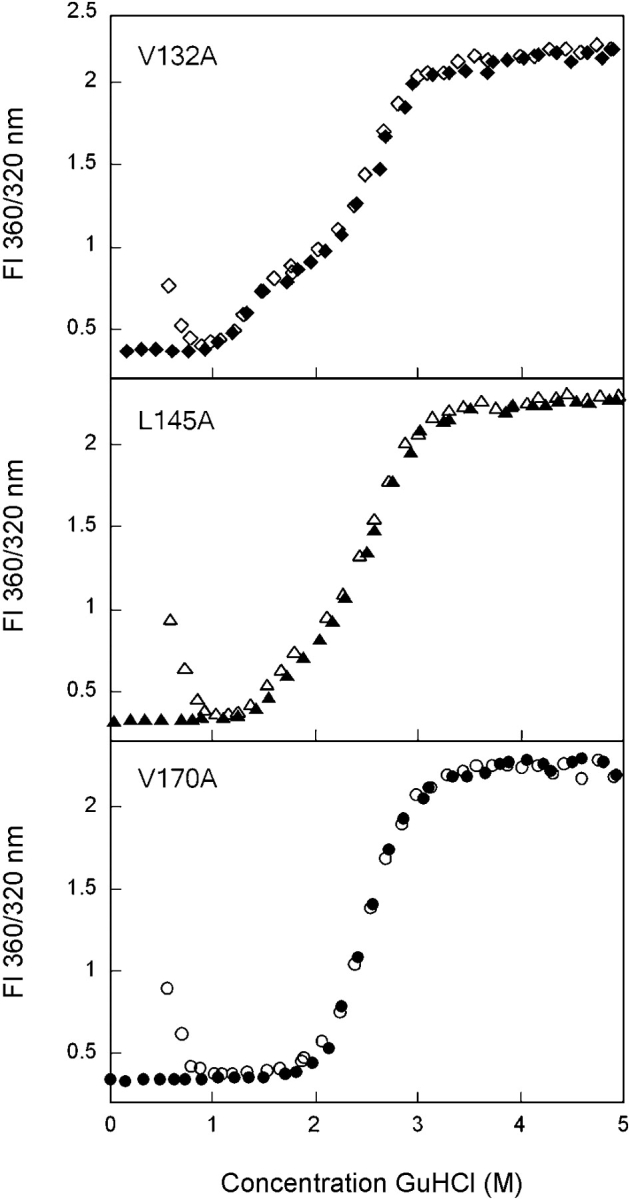
Equilibrium unfolding (closed symbols) and refolding (open symbols) of the C-terminal domain mutants V132A (♦), L145A (▴), and V170A (•). Fluorescence spectroscopy was used to probe the solvent accessibility of tryptophans in each sample using an excitation wavelength of 295 nm. Data were analyzed by fluorescence intensity at 360/320 nm. Protein was present at 10 μg/mL in 10 mM sodium phosphate, 5 mM DTT, 1 mM EDTA (pH 7.0), and GuHCl from 0 to 5.5 M. Samples were incubated at 37°C for 24 h prior to measuring fluorescence emission. Transitions of all mutants, except V170A, were best fit by a three-state model. The transitions of V170A were best fit by a two-state model.
