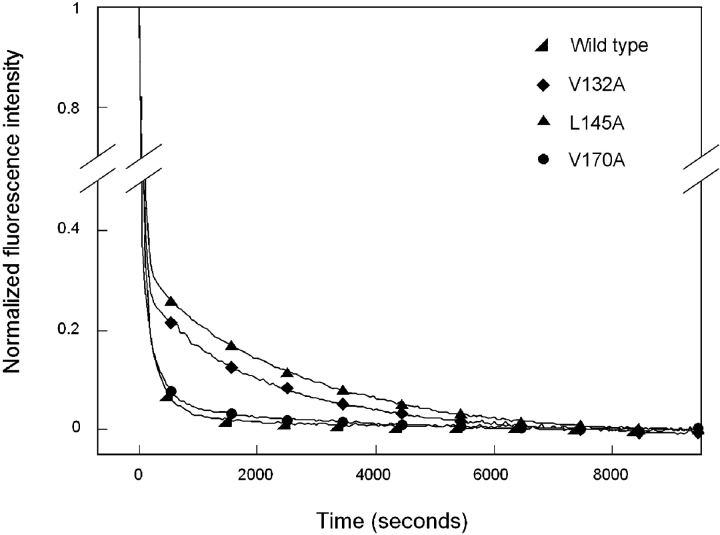
Figure 8.
Productive kinetic refolding of wild type (◂) HγD-Crys and C-terminal domain mutants V132A (♦), L145A (▴), and V170A (•). Black lines represent data points taken every 1 sec, with symbols included for ease of viewing. Fluorescence emissions were normalized for ease of viewing and comparison. The proteins were first unfolded at 100 μg/mL in 5.5 M GuHCl, 37°C for 3 h. Refolding was initiated by dilution of unfolded proteins into 10 mM sodium phosphate, 5 mM DTT, and 1 mM EDTA (pH 7.0), using a syringe injection port, to give a final concentration of 1.0 M GuHCl and 10 μg/mL protein. Structural changes during refolding were monitored by changes in fluorescence emission at 350 nm for 3 h. The temperature was maintained at 37°C during refolding using a circulating water bath. The data were fit to two exponentials to calculate rate constants. Inclusion of extra exponentials improved the fits. It was not possible to determine whether the extra exponentials characterized the genuine population of further intermediates.