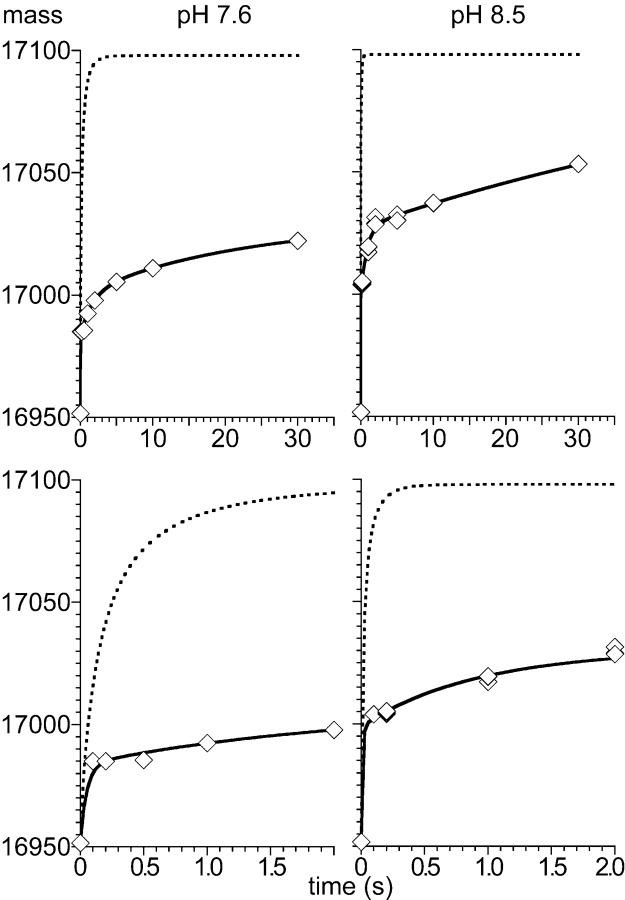
Figure 2.
Quenched-flow amide hydrogen exchange of full-length apo-myoglobin. Amide hydrogen exchange of apo-myoglobin (100 pmol) was measured at pH 7.6 (left) and 8.5 (right) for 0.1 sec to 30 sec and compared to the overall intrinsic chemical exchange (dashed curves) as determined for each amide using the HXPep program (courtesy Z. Zhang). The solid line represents the fit of a triple exponential rate equation (y = A∞− A1•exp(−k1•t) − A2•exp(−k2•t) − A3•exp(−k3•t)) to the data whereby for the fastest rate k1 the value derived from a fit to the intrinsic chemical exchange data was used. The lower panels show a zoom of the first 2 sec of the exchange reaction.