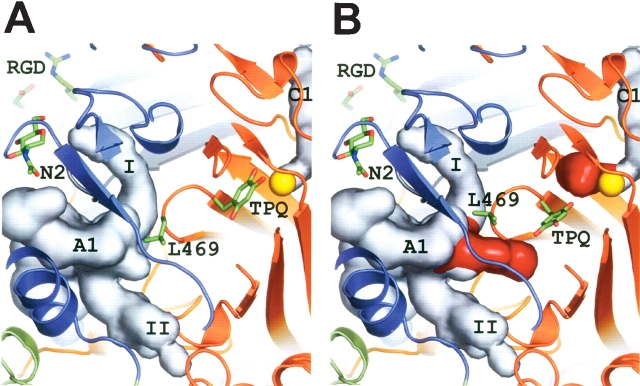
Figure 4.
The active site architecture of hVAP-1. (A) Active site in the inactive state (crystal structure) where TPQ is in the “on-copper” conformation and where L469 serves as a “guardian” blocking entry to the substrate channel. (B) Active site in the activated state (modeled) where TPQ is in the “off-copper” conformation and where the conformation of L469 has been adjusted to allow free access to the substrate channel. The side-chains of TPQ, L469, and the RGD motif, as well as the carbohydrate at site N2 are shown as sticks. The active site cavity A1 and the central cavity C1 are seen as light gray surfaces. The red surface color indicates additional space within cavities A1 and C1 in the modeled active state structure. The copper atom of the active site is shown as a yellow sphere, and the domain colors are as in Figure 1 ▶.