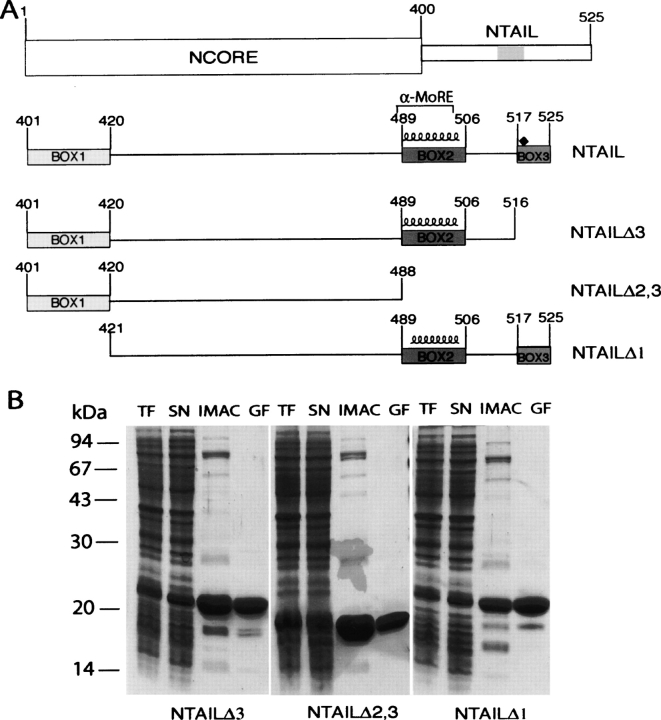
Figure 3.
(A) Schematic representation of NTAIL deletion proteins. (Top) Domain organization of N showing that it is composed of two regions, NCORE (aa 1–399) and NTAIL (aa 401–525). The epitope recognized by the anti-N mAbs, Cl 25 mAb (aa 457–476) is shaded. NTAILΔ3, NTAILΔ2,3, and NTAILΔ1 are devoid of Box3, Box2 plus Box3 and of Box1, respectively. The three NTAIL deletion proteins contain an N-terminal hexahistidine tag and a C-terminal Flag. The predicted α-helix (residues 489–504), as well as the α-MoRE (aa 488–499), i.e., the region shown to be involved in induced folding of NTAIL through binding to P (see Bourhis et al. 2004), are indicated. The position (aa 518) targeted for the Tyr → Trp substitution is highlighted by a black diamond. (B) Purification of NTAIL deletion proteins from E. coli. Coomassie blue staining of a 12% SDS-PAGE. (TF) Bacterial lysate (total fraction); (SN) clarified supernatant (soluble fraction); (IMAC) eluent from immobilized metal affinity chromatography; (GF) eluent from gel filtration.