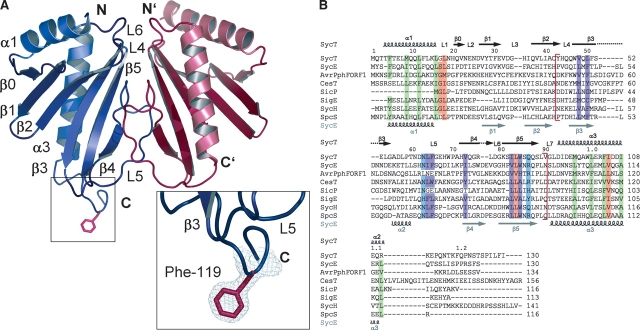
Figure 1.
(A) Ribbon diagram of the structure of Yersinia enterocolitica chaperone SycT (SycT122 crystal form #2, monomer A in blue, monomer B in red). The α-helix and β-strand numbering corresponds to that of earlier TTS chaperone structures. (Insets) The well-defined electron density of the protruding Phe119 side chain (red sticks) of monomer A. (B) Multiple sequence alignment of SycT with related TTS effector chaperones. Conserved hydrophobic residues (green) in SycT helices α1 (Phe5, Met9, Leu12, and Leu16) and α3 (Met97, Leu101, Phe104, and Ile108); SycT dimerization interface (red brackets) involves residues Tyr43, His44, Trp47, Gln49, Phe51, Asp62, Asn63, Leu64, Phe65, Trp69, Pro70, Ala71, Val73, Gly75, Arg76, Leu77, Trp84, Gln87, and Val90. The sequence section containing the dimerization-mediating residues includes also conserved polar residues (Asn63 and Gln86, in blue) and hydrophobic residues (Val48, Leu50, Leu64, Phe65, Val73, Ile82, and Trp84, in purple); other conserved residues (Gly17, Leu18, Leu83, and Ile105) are highlighted in orange. Sequences were aligned with ClustalW and adjusted by analyzing the SycT structure. Secondary structure elements for SycT are indicated above and those for SycE below the sequences. Y. enterocolitica SycT (GenBank AAD16809), Yersinia pseudotuberculosis SycE (NC_006153), Pseudomonas syringae AvrPphF ORF1 (AAF67148), Escherichia coli CesT (P58233), Salmonella typhimurium SicP (AAC38655), Salmonella enterica SigE (NP455587), Yersinia pestis SycH (NP052425), and putative chaperone Orf1 from Pseudomonas aeruginosa, abbreviated to SpcS (AAA66490).