Figure 4.
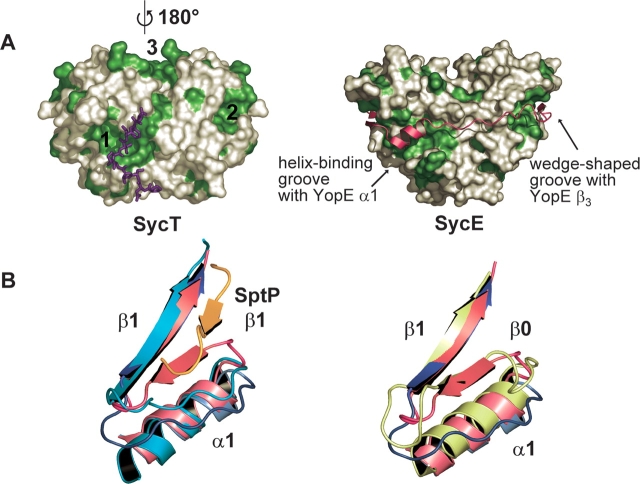
Effector-binding sites in SycT. (A) Two extended hydrophobic patches (1, 2) located on the surface of SycT (left, SycT122 crystal form #1) are duplicated by symmetry to yield four patches per dimer. In the native SycT crystal, patch 1 forms a hydrophobic interaction with residues 125–130 of the C-terminal peptide (violet sticks). Patches 1 and 2 correspond to those of a SycE dimer involved in effector binding (right; SycE in complex with the CBD of YopE [YopE23–78, red]). SycT has an additional hydrophobic patch (3) not observed in SycE. Surfaces corresponding to hydrophobic side chains are depicted in green. (B) A shallow groove alongside hydrophobic patch 2 is involved in effector binding in SicP. In SycT and Spa15, this groove is filled by the additional strand β0 formed by residues connecting α1 and β1. Both figures show the two superimposed monomers of native SycT: Monomer one (red) displays the additional strand β0, while in the other monomer (blue) β0 has changed into a loop and moved toward α1, opening a shallow groove. (Left) The SicP/SptP complex is superimposed on SycT (SicP in green, SptP in orange). In SicP, β1 and the loop connecting α1 with β1 form a groove occupied by strand β2 of the effector SptP, extending the chaperone β-sheet. Should YopT follow the same path on SycT as SptP does on SicP, it would partially overlap with strand β0 of the red SycT monomer but not with the conformationally rearranged blue monomer. (Right) Overlay of Spa15 (light green) and SycT. Although the connecting loop between α1 and β1 is four residues longer in Spa15 than in SycT, the structural organization is similar to the red SycT monomer.