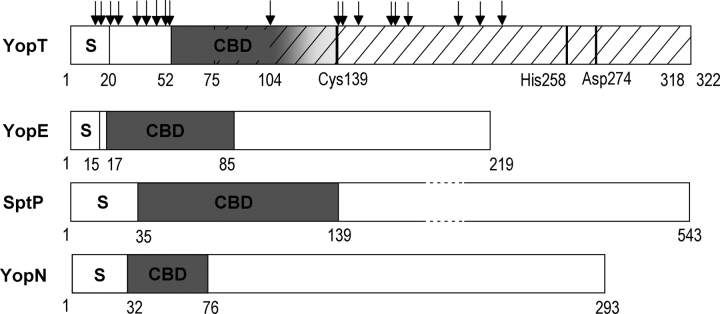
Figure 6.
Domain organization of YopT. Domain borders are designated by residue numbers. S indicates secretion signal; CBD, chaperone- binding domain. YopT: YopT region (residues 75–318, hatched) capable of substrate binding and proteolytic cleavage (catalytic triad Cys139, His258, and Asp274). Sites susceptible to protease cleavage (arrows) were identified by N-terminal sequencing of fragments produced by limited proteolysis of the complex YopT/SycT. The accumulation of sites N-terminally of amino acid 52 and C-terminally of 140 indicates the CBD to comprise at least residues 52–103. The C-terminal border of the CBD may lie after the single cleavage site at residue 104 (indicated as a gradient of gray). For comparison, the CBD of other TTS effectors are plotted below. YopE from Yersinia pseudotuberculosis, SptP from Salmonella typhimurium, and YopN from Y. pestis (Stebbins and Galan 2001; Birtalan et al. 2002; Schubot et al. 2005).