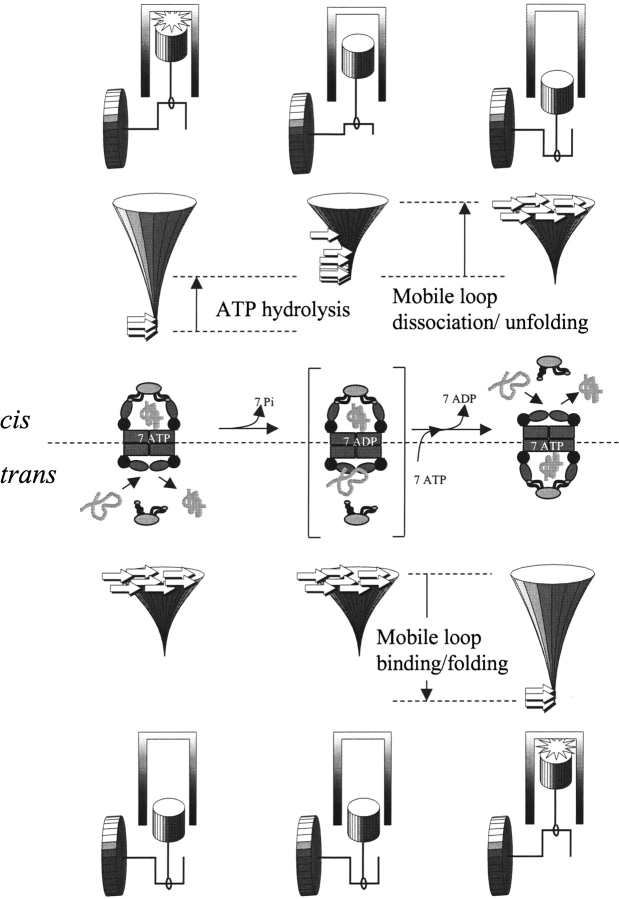
Figure 5.
Conformational and energetic events during reciprocation of the chaperonin machine. Seven ATP molecules stabilize the cis ring of subunits with apical domains up, and co-chaperonin is bound with mobile loops folded into the GroEL-bound hairpin conformation. ATP hydrolysis destabilizes the cis-ring complex with the co-chaperonin (indicated by the reshaped folding funnel). Mobile loop unfolding and co-chaperonin dissociation from the cis ring, coupled with ATP and co-chaperonin binding to the trans ring, cause the chaperonin to reciprocate to the conformation with trans subunits up and cis subunits down. The structures at the beginning and the end of the cycle are indistinguishable. For wild-type GroEL, the ADP-bound intermediate (bracketed) decays rapidly and steadily because mobile loop dissociation/unfolding (cis) and association/folding (trans) encounter no significant enthalpic barriers. Thus, the free energy of mobile-loop unfolding/folding acts like a flywheel to prevent the chaperonin machine from stalling, and the overall rate of reciprocation is controlled by the rate of ATP hydrolysis. “High-affinity” GroEL mutants stall in the ADP-bound intermediate. “Low-affinity” co-chaperonins can restore reciprocation of high-affinity GroEL mutants.