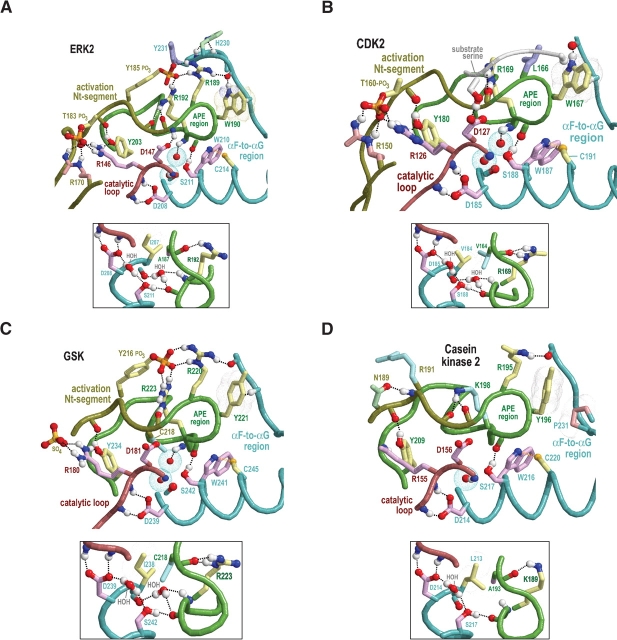
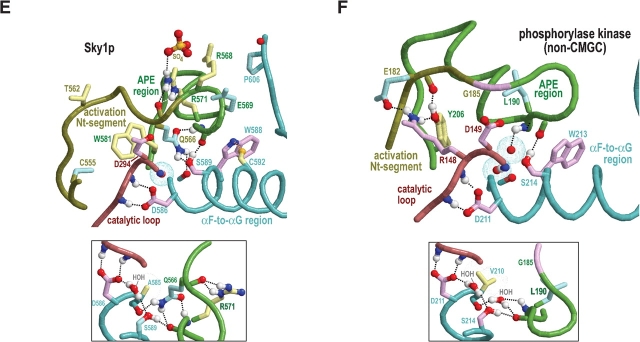
Figure 4.
Structural features surrounding buried waters located between the APE region and the catalytic loop in CMGC and other protein kinases. In the main figures, the buried waters are shown as oxygens surrounded by turquoise dot clouds; in the inset, predicted hydrogen bonds formed by these waters are shown. Residue side chains and canonical glycine main chains are colored by functional categories as follows: kinase-shared, pale magenta; CMGC-specific, light yellow; intermediate between kinase-shared and CMGC-specific, light orange to pale red; family-specific, pale cyan; intermediate between CMGC-specific and family-specific, pale green; and subfamily-specific, light blue. Hydrogen bonding carbons are colored as their corresponding side chains, and van der Waals interactions are depicted as dot clouds. See the legend to Figure 3 ▶ for other bond representations and coloring conventions; see text for details. (A) Erk2 MAP kinase (PDB 2erk). (B) CDK2 (PDB 1qmz). (C) GSK (PDB 1gng). (D) CK2α (PDB 1lp4). In CK2α the water nearer the APE region is missing, presumably due to filling of its binding cavity by the side chain of L213, which is distinctively conserved within CK2α. (E) The SRPK-related Sky1p kinase (PDB 1how). The water nearer the APE region is replaced by the side chain of Q566, which occupies the conformationally strained position in SRPKs. Note that both the nitrogen and the oxygen of the glutamine side chain participate in hydrogen bonds that, in other protein kinases, are typically established by this water. (F) The (non-CMGC) phosphorylase kinase (PDB 2phk). As shown here, these buried waters also occur in non-CMGC protein kinases.