Figure 3.
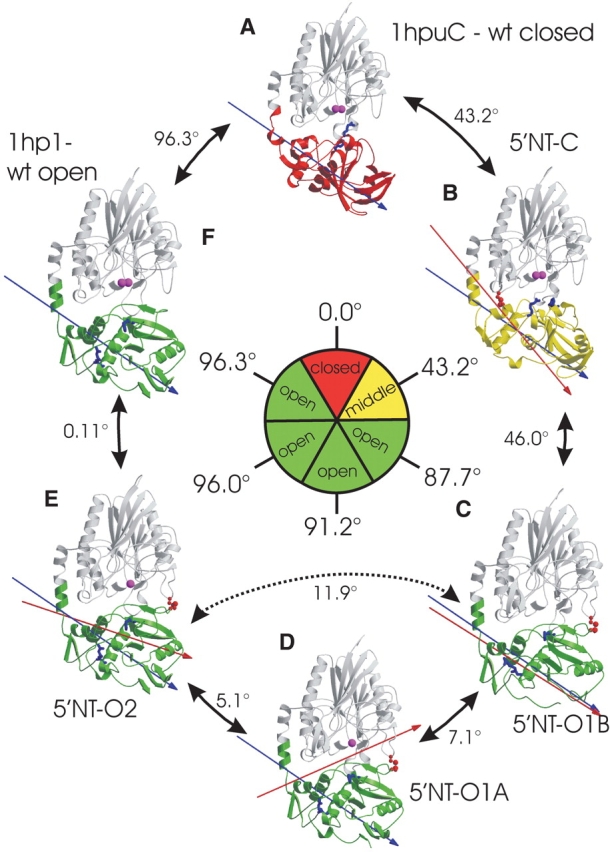
Comparison between wild-type and mutant structures, based on a superposition of the N-terminal domains, shown in gray. The moving C-terminal domains are color-coded according to their position with respect to the N-terminal domain. The closed conformation is depicted in red, and chosen as reference point (0.0°). The intermediate conformation is shown in yellow, and the open conformations, in green. Arrows between two structures indicate the degree of rotation of the C-terminal domain between these structures. The rotation of the C-terminal domain can be followed by the residues Arg 375 and Phe 498 (light-blue stick models) that belong to the binding pocket and point in the closed conformation towards the active site (indicated by the metal ions shown in magenta). The inner circle gives the degree of rotation relative to the reference structure. Because the interdomain rotation axes are not colinear, the numbers in the outer circle do not add up to the overall rotation shown in the inner circle. The engineered disulfide bonds are shown in red. The blue arrow represents the 96° axis of rotation between the most closed and the most open conformation, and the red arrow marks the interdomain rotation axis between a structure and its counterclockwise neighbor.
