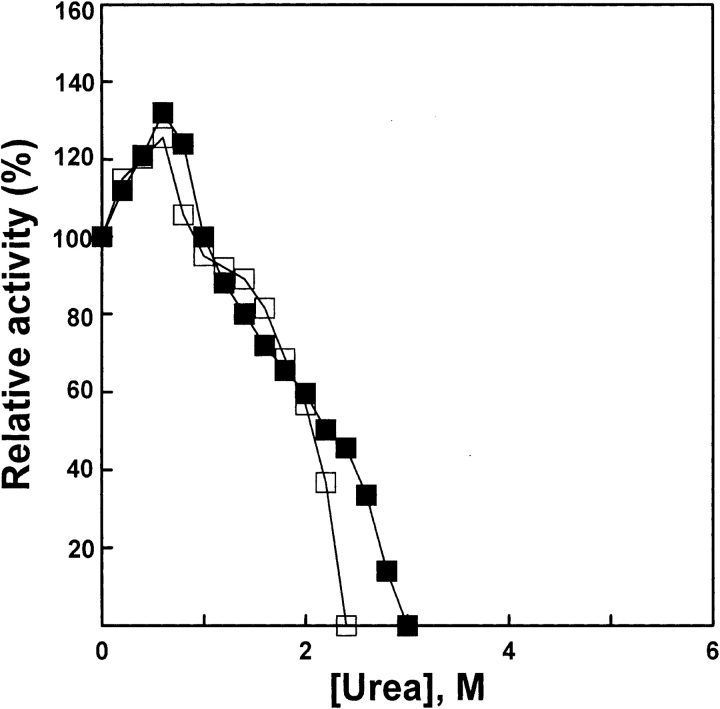
Figure 1.
Relative activity of AK. Enzyme was denatured for 1 h at 25°C in the standard buffer (0.1 M glycine-NaOH, 1 mM DDT at pH 8.6) in the presence of various concentrations of urea, and enzymatic activity (filled squares) was measured. Denatured enzyme was diluted into the standard buffer at 25°C in the presence of various concentrations of urea, and enzymatic activity (open squares) was measured after 4 h; native AK was used as control. The final urea concentrations were 0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6, 1.8, 2.0, 2.2, 2.4, 2.6, 2.8, and 3.0 M, respectively. The final enzyme concentrations of native and denatured AK were 5.3 and 3.2 μM, respectively.