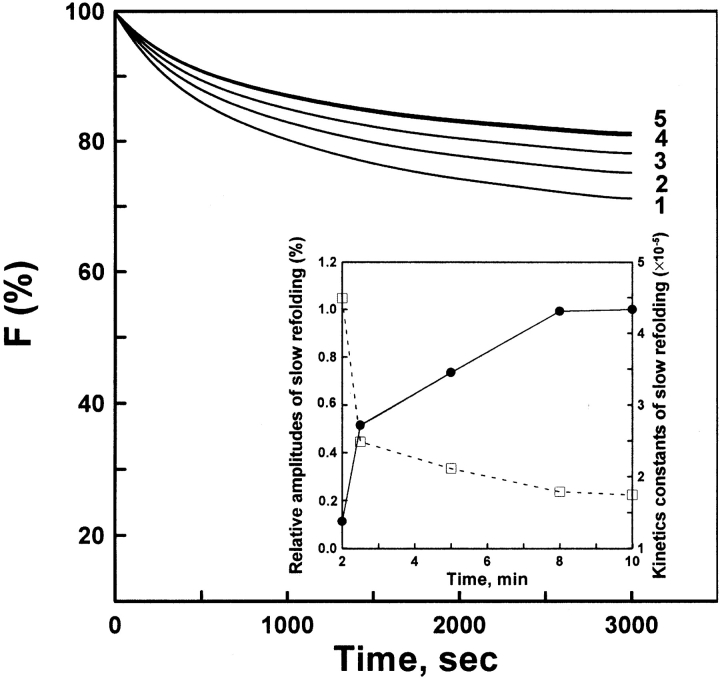
Figure 7.
Refolding assay of AK samples. The native AK was completely unfolded in 8-M urea solution for 2 min. The denatured protein of different incubation time intervals was diluted in standard buffer containing 0.4 M (NH4)2SO4. The other procedures were the same as for Figure 6 ▶. The final enzyme concentration was 4.5 μM. The incubation times were 2 (curve 1), 2.5 (curve 2), 5 (curve 3), 8 (curve 4), and 10 (curve 5) min, respectively. The inset plot shows the effects of different incubation times on the relative amplitude (filled circles) and the apparent rate (open squares) of slow phase refolding. The reactions all occurred at 5°C.