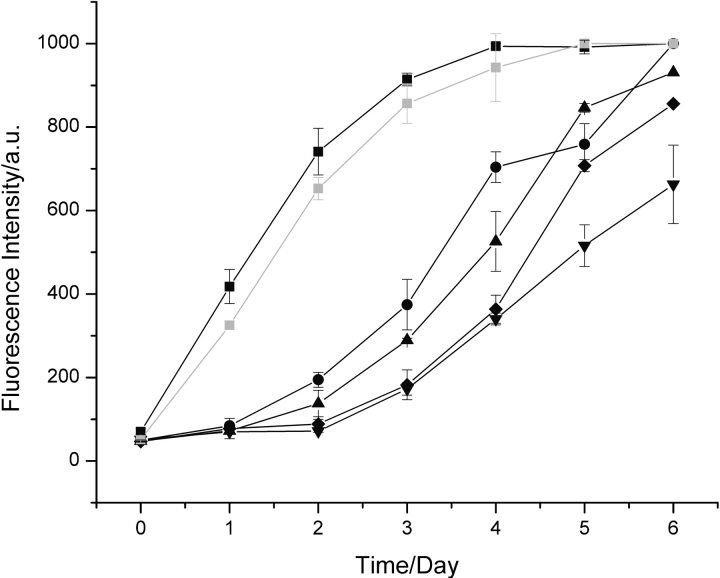
Figure 1.
Aggregation of hen lysozyme. Data are shown for the aggregation of 1 mM hen lysozyme at pH 2.0 and 65°C monitored by thioflavin-T fluorescence in the absence of added fibrils (down triangles) and following addition of 5% (v/v) (black squares), 2.5% (v/v) (circles), 1% (v/v) (triangles), and 0.5% (v/v) fibrils preformed from hen lysozyme at pH 2.0 (diamonds). Seeding with a 5% (v/v) aliquot of a solution of amyloid fibrils containing purified full-length hen lysozyme gave essentially identical kinetics (gray squares) to aliquots formed simply by incubation at pH 2.0 and 65°C in which a significant degree of fragmentation had occurred (see text).