Figure 5.
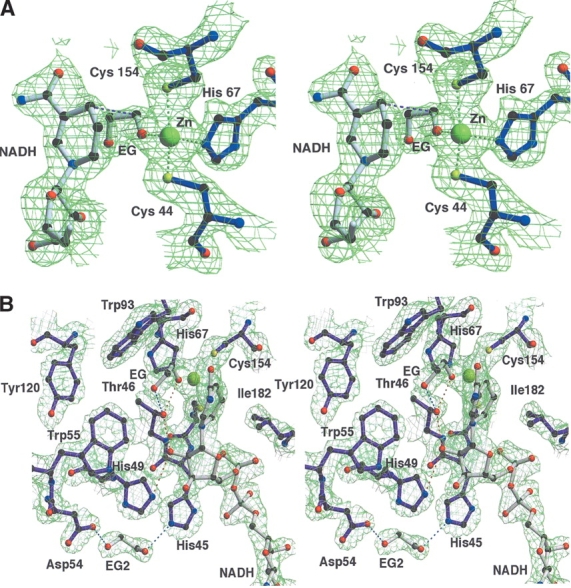
(A) Stereo view of the |2Fo-Fc| electron-density map of the catalytic site region of PaADH contoured at 1.5σ. The Zn2+ ion (in yellow) is coordinated by the sulfur atoms of Cys 44 and Cys 154, by the nitrogen of His 67, and by the oxygen of EG; the ion-ligand distances are 2.3 Å, 2.5 Å, 2.2 Å, and 2.2 Å, respectively. The distance between the C1 atom of the substrate and the C4 of NADH, assumed for the hydride ion transfer, is shown in pink (3.7 Å). For clarity, the cover-radius option was used to exclude from the map any regions placed farther than 1.8 Å from the selected atoms. (B) Stereo view of the proton-shuttling pathway of PaADH. A proton shuttles from the substrate’s EG hydroxyl group, ligated by the Zn2+ ion via Thr 46, 2′OH of the nicotinamide ribose, and His 49. The substrate is stabilized by an additional H-bond with Thr 46 (shown in blue). A second EG molecule at the proposed “proton exit” near Asp 54 is stabilized by an H-bond with His 45 and Asp 54.
