Figure 4.
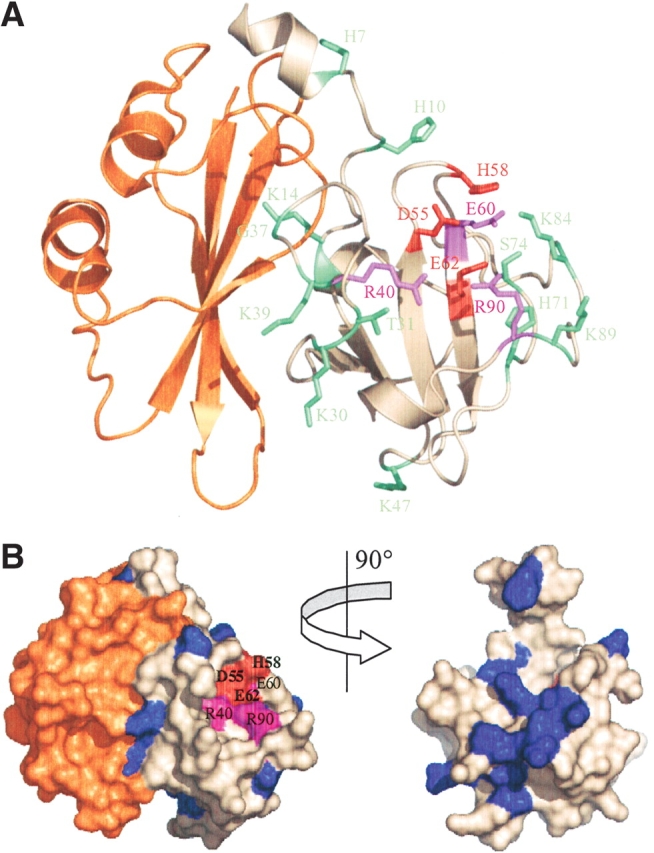
Structure of the E3 rRNase-Im3 complex highlighting the effect of alanine substitution on colicin E3 cytotoxicity and sequence conservation among rRNase-like bacteriocins. (A) Residues colored according to the effect of mutation on colicin E3 cytotoxicity; red indicates no detectable cytotoxicity, purple indicates severe impairment of cytotoxic activity, and green indicates little change relative to wild-type colicin E3 (see Fig. 1 ▶). The immunity protein (Im3) is colored orange. (B) Surface representation with E3 rRNase variable residues colored blue and conserved residues shown in white. The active site residues D55, H58, and E62 (all conserved) are colored red, and R40, E60, and R90 are colored purple. The right figure shows the E3 rRNase domain alone rotated ~90° to show sequence variability at the E3 rRNase-Im3 interface. This figure was prepared using the program Pymol v2.1 (PDB code 1E44; Carr et al. 2000).
