Figure 8.
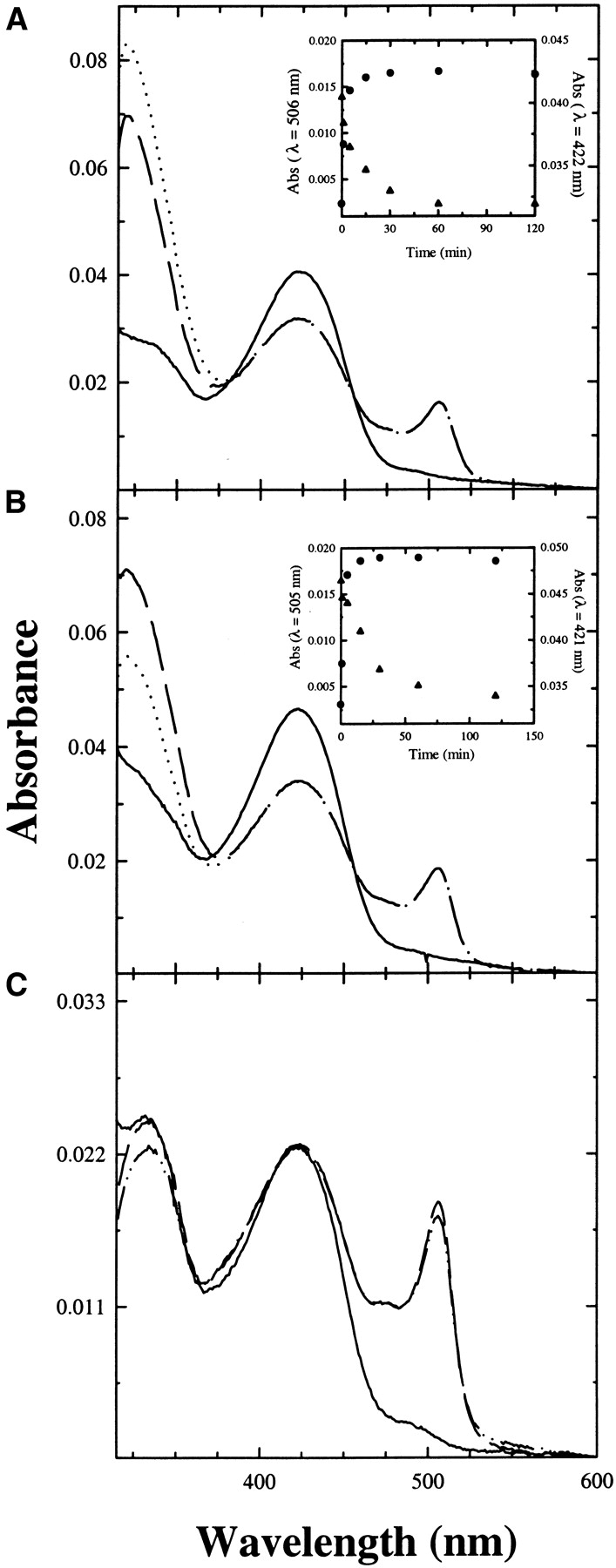
Reactivity of Trpase-doped gels and Trpase in solution with L-tryptophan. Absorption spectra of Trpase-doped gels were recorded in a solution containing 50 mM potassium phosphate (pH 7.5) at 25°C, (A) in the absence (solid line) and presence of 10 mM L-tryptophan after 120 min of reaction (dashed line), and after subtraction of pyruvate absorbance (dotted line), (B) in the absence (solid line) and presence of 10 mM L-tryptophan and 5 mM benzimidazole, after 120 min of reaction (dashed line), and after subtraction of pyruvate absorbance (dotted line). Pyruvate concentration was evaluated by a coupled lactate dehydrogenase assay (see Materials and Methods). Insets: (A) Absorbance changes at 506 nm (•) and 422 nm (▴) as a function of time; (B) absorption changes at 505 nm (•) and 421 nm (▴) as a function of time. (C) Absorption spectra of Trpase in a solution containing 50 mM potassium phosphate (pH 7.5) at 25°C in the absence (solid line), and presence of 10 mM L-tryptophan (dashed line) and 10 mM L-tryptophan and 5 mM benzimidazole (dashed/dotted line). Spectra were corrected for the contribution of pyruvate absorption.
