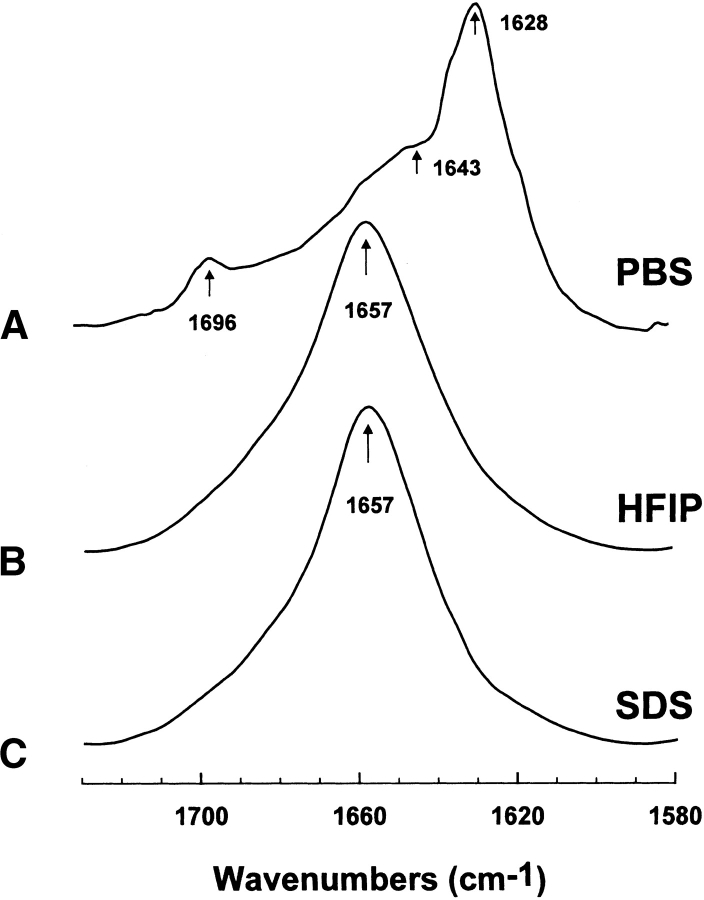
Figure 2.
Fourier transform (FTIR) spectra of the amide I band of FP in phosphate-buffered saline (PBS), hexafluoroisopropanol (HFIP), and sodium dodecyl sulfate (SDS) at 25°C, as described in Materials and Methods. (A) FP concentration was 470 1μM in deuterated PBS (pH 7.4). The arrows at 1628 and 1696 cm−1 denote a β-sheet component, while the shoulder at 1643 cm−1 also indicates random structure (Gordon et al. 2002). (B) FP concentration was 470 μM in deuterated hexafluoroisopropanol (HFIP)/water/formic acid (70:30:0.1, v/v; Gordon et al. 2002). (C) FP concentration was 470 μM in 94 mM SDS and deuterated PBS (pH 7.4) at a peptide/lipid (P/L) ratio of 1 : 200. The arrows at 1657 cm−1 in B–C indicate a dominant α-helix component for FP in these membrane-mimic environments. Spectra have been normalized for comparison. The abscissa for each spectrum (left to right) is 1730 to 1580 cm−1, while the ordinate represents absorption (in arbitrary units).