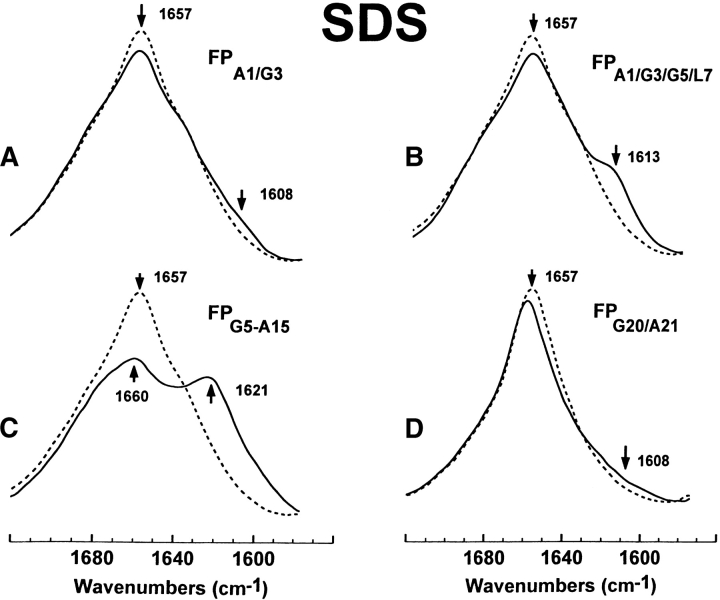
Figure 3.
FTIR spectra of the amide I band for the 12C-carbonyl (i.e., “native”) FP peptide and a suite of multiply 13C-carbonyl enhanced FP peptides in sodium dodecyl sulfate (SDS; Fig. 1 ▶). Peptides were suspended at 470 μM in 94 mM SDS and deuterated PBS at a peptide/lipid (P/L) ratio of 1 : 200. Spectra were recorded at 25°C on the peptide, which was first dried on the ATR from the SDS suspension, and then resolvated with deuterated PBS: (A) FPA1/G3 is the solid line and native FP is the dashed line. The amide I band is shown for the native FP spectrum, with a dominant α-helical component centered at 1657 cm−1. The minor peak at 1608 cm−1 in the FPA1/G3 spectrum indicates random structure. (B) FPA1/G3/G5/L7 (solid line), FP (dashed line). The minor peak at 1613 cm−1 in the FPA1/G3/G5/L7 spectrum indicates a mix of α-helical and random components. (C) FPG5-A15 (solid line), FP (dashed line). The major peak at 1621 cm−1 in the FPG5-A15 spectrum indicates a strong α-helical component. (D) FPG20/A21 (solid line), FP (dashed line). The broad, minor shoulder centered at 1608 cm−1 and extending to ~1590 cm−1 in the FPG20/A21 spectrum indicates random structure and minor β-sheet.