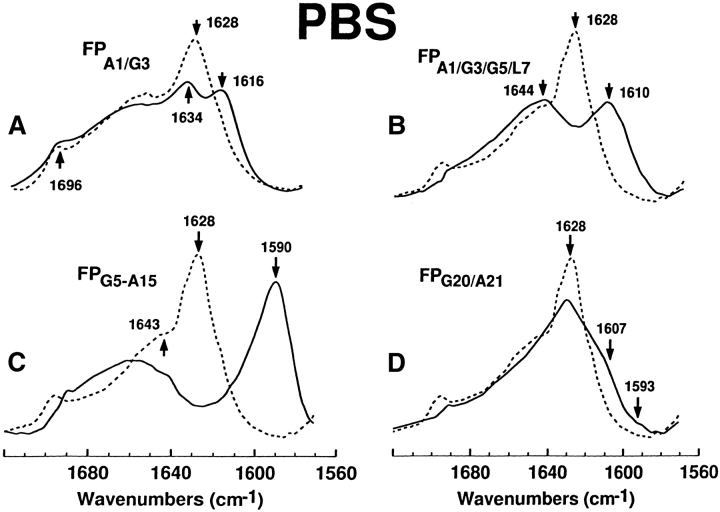
Figure 6.
FTIR spectra for the 12C-carbonyl (i.e., “native”) FP peptide and a suite of multiply 13C-carbonyl enhanced FP peptides in PBS solution. Peptides were suspended at 470 μM in deuterated PBS (pH 7.4), as described in Materials and Methods. (A) FPA1/G3 is the solid line and native FP is the dashed line. The amide I band is shown for the native FP spectrum, with a dominant peak at 1628 cm−1 and a minor peak at 1696 cm−1 denoting antiparallel β-sheet, and a high-field shoulder at 1643 cm−1 indicating random structure. The FPA1/G3 spectrum indicates a residual 12C amide peak at 1634 cm−1 and a 13C amide peak at 1616 cm−1, denoting antiparallel β-sheet. (B) FPA1/G3/G5/L7 (solid line), native FP (dashed line). The FPA1/G3/G5/L7 spectrum indicates a residual 12C amide peak at 1644 cm−1 and a 13C amide peak at 1610 cm−1, denoting antiparallel β-sheet. (C) FPG5-A15 (solid line), native FP (dashed line). The FPG5-A15 spectrum indicates a minimal residual 12C amide peak at and a pronounced 13C amide peak at 1590 cm-1, denoting antiparallel β-sheet. (D) FPG20/A21 (solid line), FP (dashed line). The low-field shoulder at ~1607 and tail at ~1593 cm−1in the FPG20/A21 spectrum indicates random (disordered) and β-sheet structure.