Figure 7.
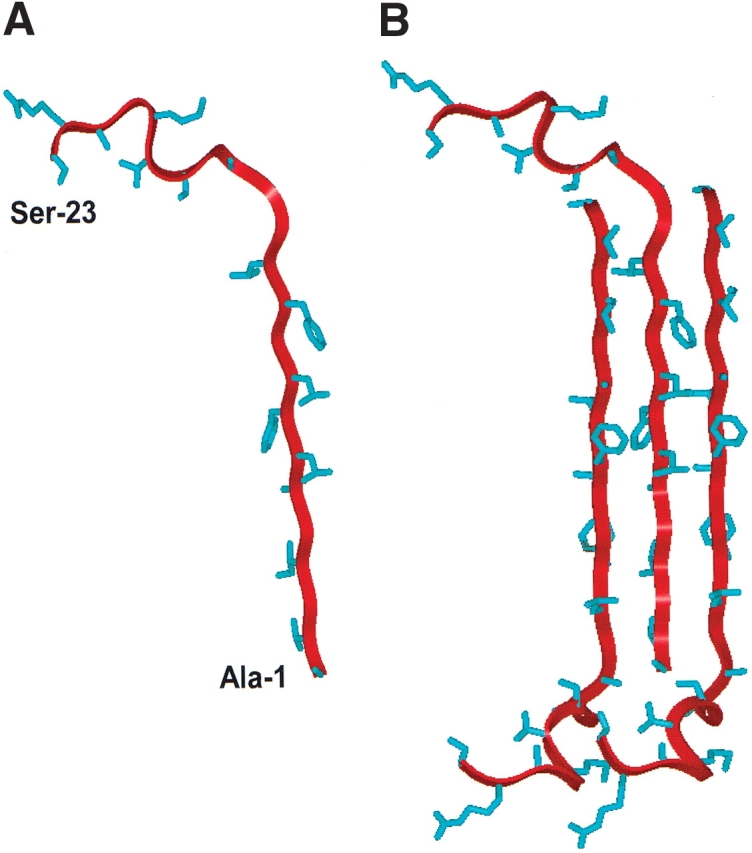
Ribbon representations of the N-terminal (FP) of HIV-1 gp41 in PBS solution as a monomer (A) and as a trimeric (B) antiparallel β-sheet. Residue-specific information on backbone conformations was derived from 13C-labeled FP peptides in the PBS solution (Figs. 4 ▶,5A–C ▶,6 ▶). Amino acid residues Ala−1to Leu-12 were determined as antiparallel β-sheet, while residues Gly-13 to Ser-23 were here assigned random and extended β-conformations (Fig. 4 ▶). (A) Monomer FP in PBS, with the conformer backbone modeled as a red ribbon and side chains as blue sticks; the N-terminal residue (Ala-1) and C-terminal residue (Ser-23) are indicated. (B) Trimeric FP in PBS with residues Ala-1 to Leu-12 as an antiparallel β-sheet, with the middle monomer assuming the same orientation as that of FP in Figure 7A. The precise vertical alignment for the monomers in this 3-sheet is undetermined.
