Figure 9.
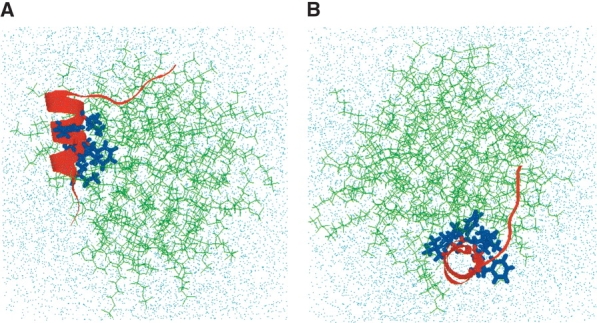
Final conformation of hydrated FP-SDS micellar system after molecular dynamics simulations, viewed from the side (A) and down (B) the peptide helix (FP residues 4–15). (A) Side view of the final configuration of the hydrated FP-SDS micelle, with the FP moving to one end of the micelle. The central core of FP remains buried in SDS, with the primary hydrophobic interactions with the micelle coming from the side chains of Ile-4, Leu-7, Phe-8, and Leu-12. However, the opposing residues on the amphipathic helix (i.e., Gly-5, Ala-6, Gly-10, Gly-13 Ala-14, Ala-15), and also residues 1–4, interact favorably with water molecules. The hydrophilic C-terminal region (residues 17–23) still lies on the micellar surface exposed to solvent, but now FP residues Ser-17 to Gly-20 form a type-1 β-turn. The SDS detergent lipids are represented by green stick models; the FP backbone, as a red ribbon with the side chains for Ile-4, Leu-7, Phe-8, Leu-9, Phe-11, and Leu-12 as blue stick models; and the water molecules and sodium and chloride ions, by dots. (B) Top view of the same final configuration for the FP-SDS micellar system, indicating that the spherical micelle has been largely compacted by the presence of FP to form a more oblate conformation.
