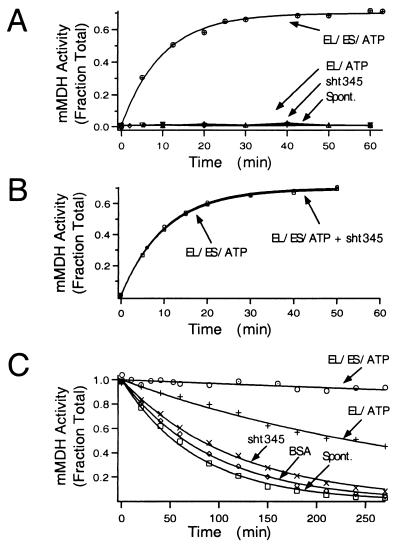
Figure 4.
Minichaperone does not assist the folding of mMDH. (A) Effect of minichaperone on folding of mMDH. mMDH denatured in 3 M guanidine-HCl was rapidly diluted (100-fold) into buffer C containing the indicated chaperones. The concentration of mMDH in the folding reactions was 0.5 μM. At the indicated times, an aliquot was removed and the yield of mMDH activity measured. mMDH activities are reported as the fraction of the input material. The following concentrations of chaperones were present in the reactions: 28 μM GroEL and 42 μM GroES protomer (EL/ES/ATP); 28 μM GroEL protomer (EL/ATP); 28 μM sht-GroEL191–345 (sht345); 0 μM GroEL (Spont.). Where indicated, 10 mM ATP was present. All mMDH experiments were carried out at 37°C. (B) Effect of sht-GroEL191–345 minichaperone on GroEL-GroES-mediated folding of mMDH. GroEL-GroES folding reactions were carried out as in A in the presence or absence of 28 μM minichaperone as indicated. (C) The effect of chaperones on thermostability of native mMDH. Native mMDH was diluted into buffer C containing the indicated chaperones. The final concentration of mMDH is 0.5 μM. The concentration of BSA was 100 μg/ml, and the concentrations of the other chaperones and ATP were as in A.