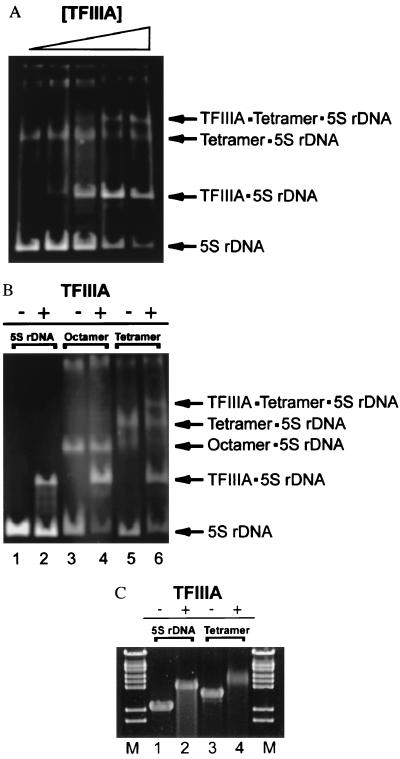
Figure 4.
Binding of TFIIIA to 208-12 DNA complexed with H3/H4 tetramers or histone octamers. (A) Binding of TFIIIA to partially saturated tetramer⋅DNA complexes. Increasing amounts of TFIIIA (0.5–3.75 μg) was incubated with 1.5 μg of n = 6 208-12 tetramer arrays and digested with EcoRI, which cuts at the junction of each 5S repeat (see Experimental Procedures). The digestion products subsequently were resolved on a 5% native polyacrylamide gel at 15 mA, constant current. (B) Comparison of the binding of TFIIIA to nucleosomal arrays and H3/H4 tetramer arrays. Naked 208-12 DNA, n = 6 208-12 nucleosomal arrays, and n = 6 H3/H4 tetramer arrays (1.5 μg each) were incubated with 3.75 μg of TFIIIA and digested with EcoRI. Samples were electrophoresed on a 5% native polyacrylamide gel as in A. Lanes 1, 3, and 5 correspond to naked DNA, nucleosomal arrays, and H3/H4 tetramer arrays incubated in the absence of TFIIIA, respectively. Lanes 2, 4, and 6 correspond to the same samples incubated with TFIIIA. (C) Agarose gel electrophoresis of TFIIIA⋅DNA and TFIIIA⋅H3/H4 tetramer array complexes before EcoRI digestion. Naked 208-12 DNA (DNA) and n = 12 H3/H4 tetramer arrays (Tetramer) were incubated with saturating levels of TFIIIA as described, followed by electrophoresis for 3 h at 60 V (constant volts) on a 0.8% agarose gel buffered with 20 mM Tris-borate (pH 7.5). Lanes 1 and 3 correspond to naked DNA and n = 12 208-12 tetramer arrays incubated in the absence of TFIIIA, respectively. Lanes 2 and 4 correspond to naked DNA and n = 12 208-12 tetramer arrays incubated with TFIIIA, respectively. λ DNA–BstEII digest was used as a marker (M).