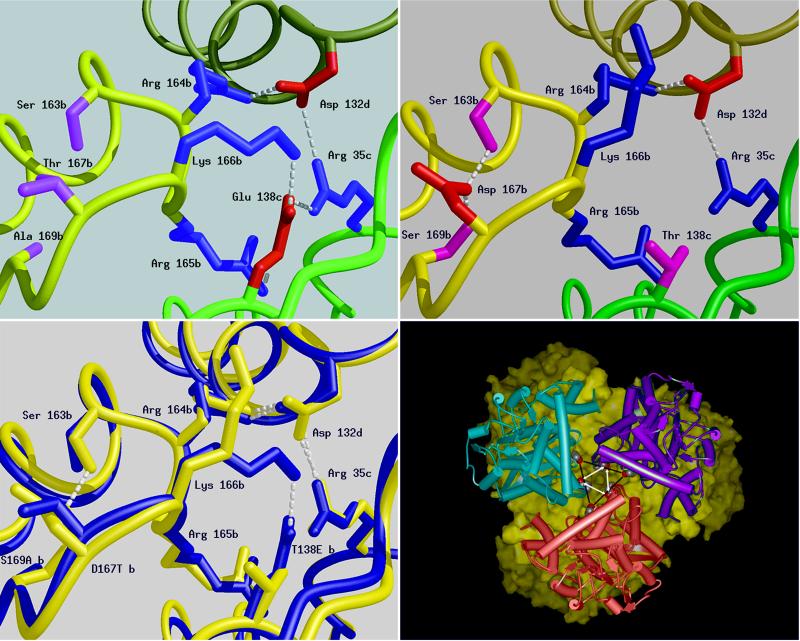
Figure 2.
Schematic diagrams of a six residue-charged cluster in the structure of the GluDHs formed between residues from three adjacent subunits produced by the program midas (31). The main chain of each subunit is shown as a smooth ribbon with b, c, and d subunits colored yellow, green, and khaki, respectively. The positively charged side chains are shown in blue, the negatively charged ones in red, and hydrogen bonds shown as dashed lines. (Upper Left) P. furiosus GluDH: Glu-138, subunit c can be seen to form triple ion-pair interactions to Arg-35, subunit c; Arg-165, subunit b; and Lys-166, subunit b. (Upper Right) The same cluster in T. litoralis GluDH, highlighting the difference caused by the substitution in this enzyme of Glu-138 by Thr (shown in pink). The main consequence of this cluster is the reduction in the size of the charged network from six residues to three. The transposition of residues 138 and 167 leads to very little change in the position of neighboring residues with the notable exception of K166, which in T. litoralis is found extended toward the inter-trimer interface rather than between the subunits of the trimer. D167 (T. litoralis) is unshielded and its carboxyl groups are buried away from the lumen on the threefold axis. (Lower Left) A superimposition of the structures of the GluDHs from T. litoralis (blue) and P. furiosus (red). This diagram highlights the difference between the two structures in the region of the sequence change at position 138. Subtle displacement of the main chain occurs with the two backbone loops of the P. furiosus GluDH further apart at position 167. (Lower Right) View along the threefold axis of T. litoralis GluDH hexamer was generated by using msi WebLab. One trimer is depicted as a solid surface (yellow) and the other as three monomers (pink, blue, and purple). The mutation sites side chains are indicated by the vertices of the central triangles (T138 black bars, D167 white arrows). The corresponding sites on the surface of the other trimer are juxtaposed.