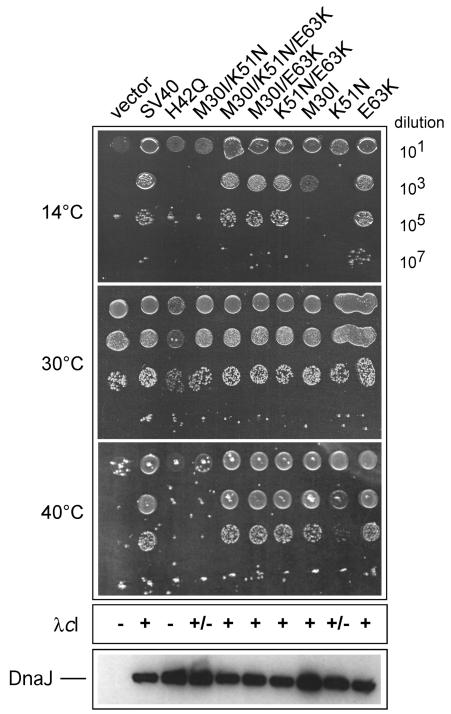
FIG. 2.
Analysis of SV40 J-domain mutant phenotypes in vivo. The WKG190 E. coli reporter strain lacks both dnaJ and cbpA genes, which renders it viable at 30°C but unable to propagate at both low and high temperatures, nor is it able to support the growth of bacteriophage λ at any temperature (16). Complementation of these genetic defects requires a functional source of DnaJ or a DnaJ chimera that has been engineered to contain a heterologous J domain. The l-arabinose-inducible vector pBAD22 or pWKG90 (SV40 J domain inserted in DnaJ in place of the native J domain) and its derivatives with the indicated SV40 J-domain mutations are shown. Fixed aliquots (5 μl) of serial dilutions of bacterial cultures grown at permissive temperature (30°C) were spotted on plates and grown at the indicated temperatures. The relative efficiency of colony formation can be directly determined by counting viable cells. The assay is sensitive for qualitative comparison but cannot be used to conclude relative activity. The efficiency of bacteriophage λcI plaque-forming ability is indicated as + if equivalent to that of wild-type bacteria (i.e., titer on test bacteria divided by titer on wild-type bacteria is ≈1), +/− if partial activity (<10−5 or five orders of magnitude less efficient plaque formation than that for the control), or − if <10−5 compared to control activity. λdnaJ+-transducing bacteriophage was used in every experiment as an infection control. An immunoblot analysis of whole-cell extracts showing the steady-state expression levels of the wild-type and mutant DnaJ proteins is presented at the bottom.