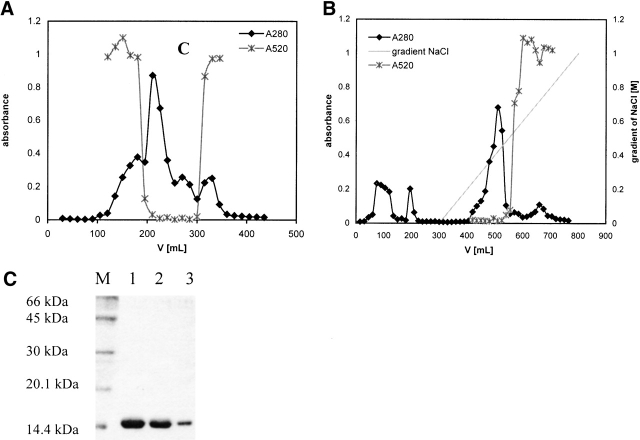
Figure 2.
(A) Gel-filtration of the H75W variant of recombinant human stefin B using Sephacryl-100 size-exclusion chromatography (SEC) column. Protein content was followed at 280 nm (filled diamonds, black line). Fractions with inhibitory activity against papain were collected and used in additional purification steps. (x) Represents inhibitory activity of the mutant H75W. (B) Cation exchange chromatography of the H75W variant of recombinant human stefin B on a SP-Sepharose fast flow column. Protein content was followed at 280 nm (black line, filled diamonds). Fractions with inhibitory activity against papain (x) were pooled and used for further application. (C) SDS-PAGE analysis of the purified site-specific mutants of the recombinant human stefin B after cation exchange chromatography. Lane 1, P36G; lane 2, P79S; lane 3, H75W; and lane M, molecular mass markers (kD).