Figure 3.
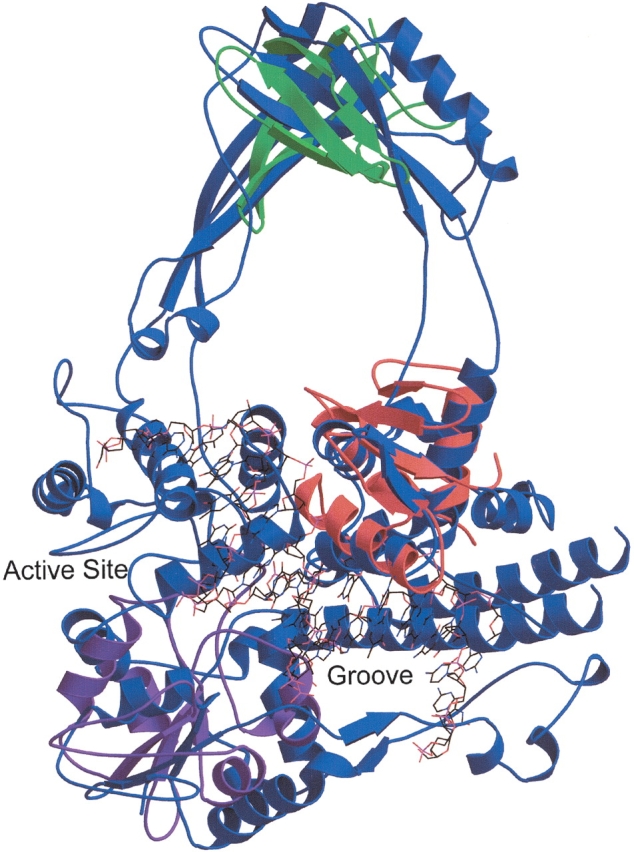
Topoisomerase III contains several small domains. A structural similarity search using DNA topoisomerase III of E. coli (SCOP domain d1d6ma_) as the query reveals local structural homology with the winged helix DNA-binding domain superfamily. The alignment of the topoisomerase (blue) to chain E of the transcription factor PU.1 of Mus musculus (SCOP domain d1puee_, shown in red), which is complexed with DNA (shown in wireframe), lends support to the hypothesis that the DNA could bind in the groove identified by Mondragon and DiGate (1999). The DNA reaches the active site of the topoisomerase as well. Repeated applications of the structural similarity search that exclude the SSEs of motifs already identified lead to the discovery of the additional motifs described in the text. The alignment of the topoisomerase to the C-terminal (UDP-binding) domain of UDP-glucose dehydrogenase (SCOP domain d1dlja3) is shown in purple, and an alignment of the major cold shock protein (SCOP domain d1c9oa_) is shown in green. This figure was produced by Molscript and rendered by Raster3D (Kraulis 1991; Merritt and Bacon 1997).
