Figure 1.
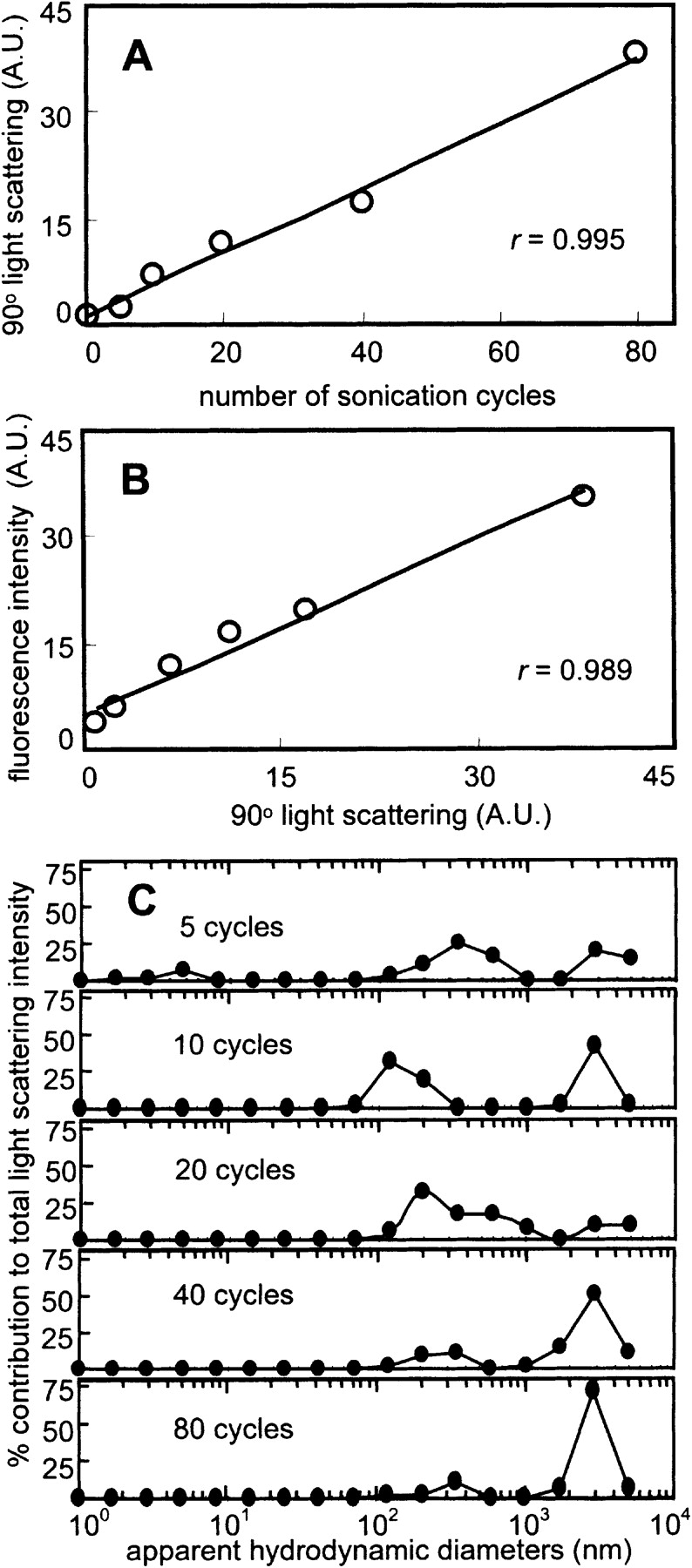
(A) Cycle number-dependence of sonication-induced protein aggregation of BSA. Light scattering increased in a linear fashion with increasing number of sonication cycles from 0 to 80 cycles (r = 0.995). (B) Correlation between ThT fluorescence and light scattering for BSA. ThT fluorescence increased in a strong linear fashion with amount of protein aggregate as determined by light scattering (r = 0.989). (C) Distribution of apparent hydrodynamic diameters of BSA as a function of sonication cycle number as determined by DLS. The unsonicated protein solution had a relatively low polydispersity with an effective diameter of ~3 nm, as expected for monomeric BSA. At 5 cycles, correlation function deconvolution shows the persistence of a distribution corresponding to monomeric BSA along with a larger aggregate distribution (>100 nm). The range of sizes is relatively unchanged between 10 and 80 cycles.
