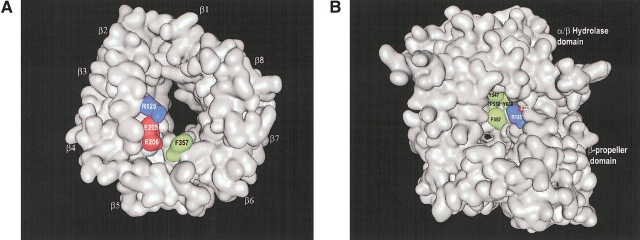
Figure 2.
(A) Surface representation of the β-propeller domain only, showing the propeller opening to the active site. The view was taken from the interface with the α/β-hydrolase domain and down the pseudo-eightfold axis. The four-strand antiparallel β-sheets of the eight blades are indicated (β1–β8). (B) Surface representation of whole DPPIV molecule, showing the side opening to the active site. Residues of DPPIV that make direct molecular interactions with tNPY are colored in both panels. Hydrophobic negatively charged and positively charged residues are shown in green, in red, and in blue, respectively. The figures were made with the program MOE (MOE, Chemical Computing Group).