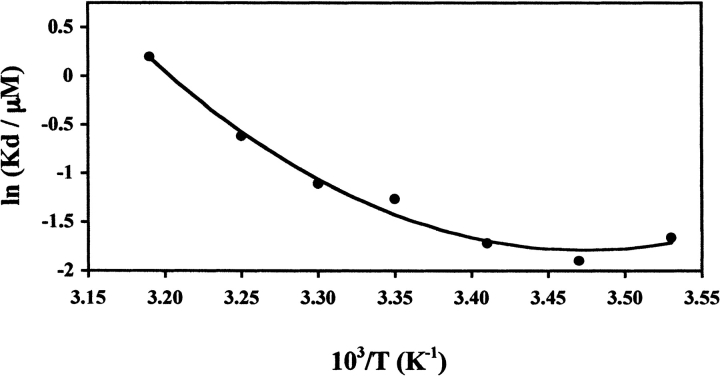
Figure 6.
Dissociation constant of the NAD+ synthetase dimer (Kd) in the presence of 2.5% (v/v) DMSO as a function of the reciprocal of temperature. The smooth curve is a fit for the data, using a simple van’t Hoff analysis (equation 14; see Materials and Methods for details). The van’t Hoff enthalpy (ΔHv1) from the fit is 113.3 (±10) kJ/mole at T = 39.0°C and the change in heat capacity (ΔCp1) is 4.67(±0.8) kJ/(mole-K). Each data point represents the Kd calculated from a global fit of six different experimental runs at temperatures 15°, 20°, 25°, 35°, and 40°C, and three different runs at 10° and 30°C. The S.D. of each data point is smaller than the diameter of the symbol.