Figure 3.
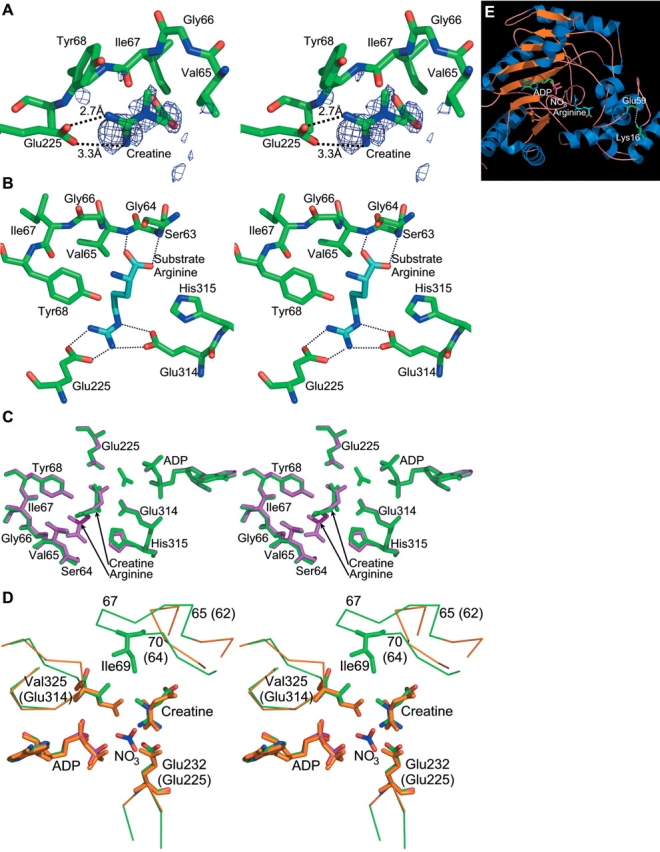
The phosphagen binding site. (A) Creatine bound in the active site of Limulus arginine kinase. The electron density shown in stereo is of a Fo−Fc sigma-A weighted difference electron density map, contoured at 1.9 σ. The map was calculated before creatine had been added to the model. Although maps later in the refinement were of higher quality, this one has had no opportunity for the introduction of phase bias, so the distinctive protrusion for the creatine methyl group is confirmation of the contents of the active site. For clarity, only a selection of residues from the neighboring large and small domain specificity loops is shown. (B) Interactions of arginine with arginine kinase in its transition state analog complex (red [Zhou et al. 1998; Yousef et al. 2002]), for comparison with the creatine interactions shown in panels A and C. Probable hydrogen bonds are shown with dotted lines. (C) The arginine kinase active site with structures of the arginine and creatine transition state like complexes superimposed. The arginine complex is shown in purple, the creatine complex in green. The creatine complex was crystallized with ATP but no nitrate. Thus, after apparent hydrolysis in the crystal, there is nothing occupying the site of the γ-phosphate. The most noticeable difference between the two structures is rotation of the substrate guanidinium by ~30 degrees. (D) Comparison of the arginine kinase-creatine-ADP active site (orange carbons) with the creatine kinase transition state analog structure (green carbons [Lahiri et al. 2002]). Again, the main difference is rotation of the guanidinium group. Arginine kinase residues are labeled with parentheses, creatine kinase, without. (E) Location of the E59-K16 salt bridge with respect to the active site. The transition state analog structure (Zhou et al. 1998; Yousef et al. 2002) is shown in ribbon form. In ball-and-stick form are the substrate analog atoms, and residues 16 and 59, which form the salt bridge.
