Figure 2.
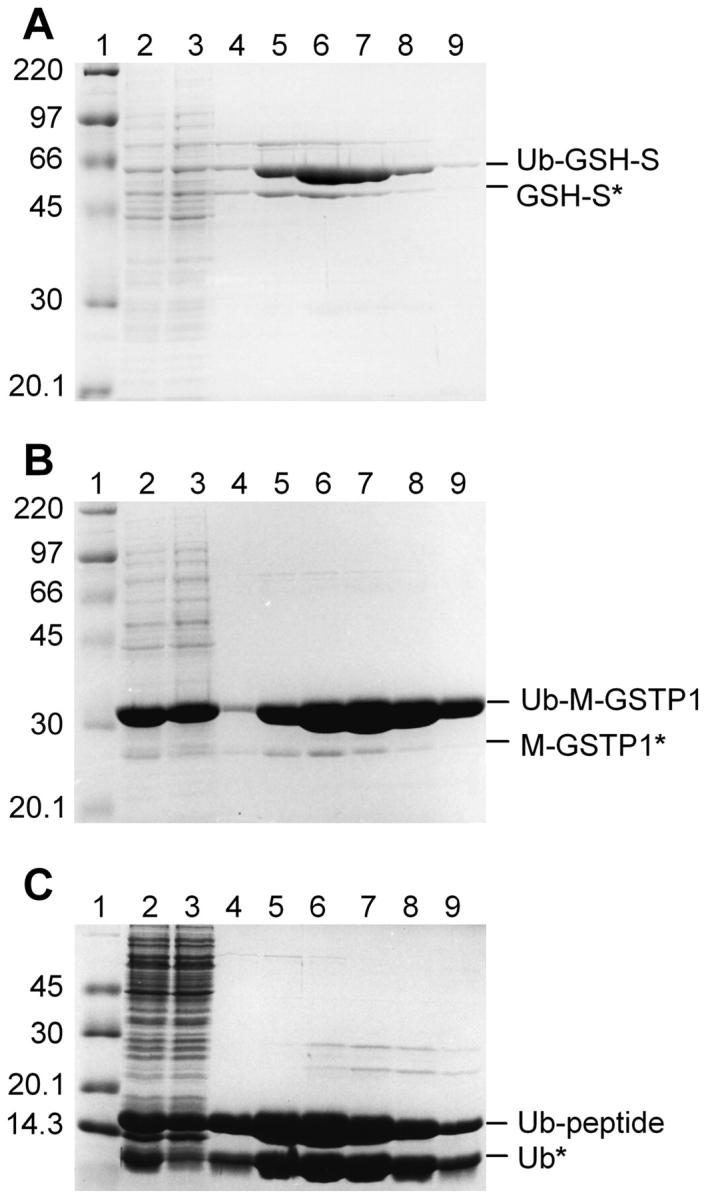
Purification of His-tagged Ub fusion proteins. Ub fusion proteins were isolated from crude E. coli extract by Ni-affinity chromatography under native conditions. Samples from sequential steps in the purification were resolved by 10% SDS-PAGE and stained with Coomassie blue. (A) Ub–GSH-S; (B) Ub–M-GST; (C) Ub-peptide. Molecular mass marker (lane 1); crude E. coli extract (lane 2); unbound proteins (lane 3); and elutions with 50 mM (lane 4), 100 mM (lane 5), 150 mM (lane 6), 200 mM (lane 7), or 250 mM (lanes 8,9) imidazole. Proteins migrating at the expected molecular mass of the Ub fusion are indicated on the right. Proteins with an asterisk represent apparent cleavage by an unknown E. coli protease (see text).
