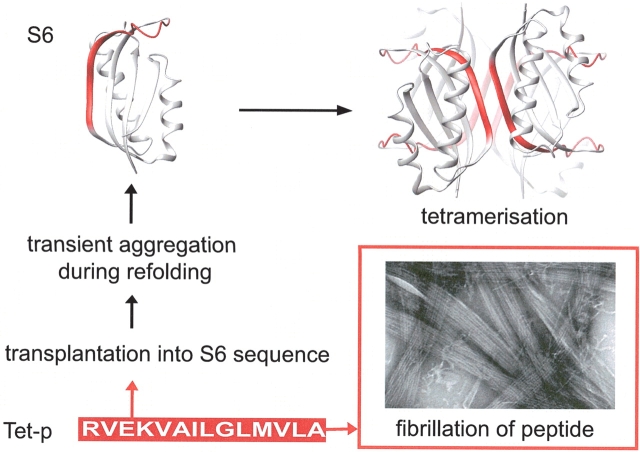
Figure 1.
The Tet-p peptide is homologous to the C-terminal part of the Aβ-peptide (aa 25–38) and displays a complex aggregation behavior, both on its own and upon insertion into other soluble proteins. Transplantation of the Tet-p peptide into the sequence of the ribosomal protein S6 triggers transient aggregation of the coil state during refolding. The transient aggregates are short-lived (20 msec) and ripped apart upon refolding of the S6 structure. On a longer time scale, however, the Tet-p sequence (red) promotes ordered tetramerization of the S6 monomers by the formation of intermolecular β-strands that are antiparallel. The different species accumulating in the fibrillation process of the free Tet-p peptide are show in Fig. 2 ▶.