Figure 1.
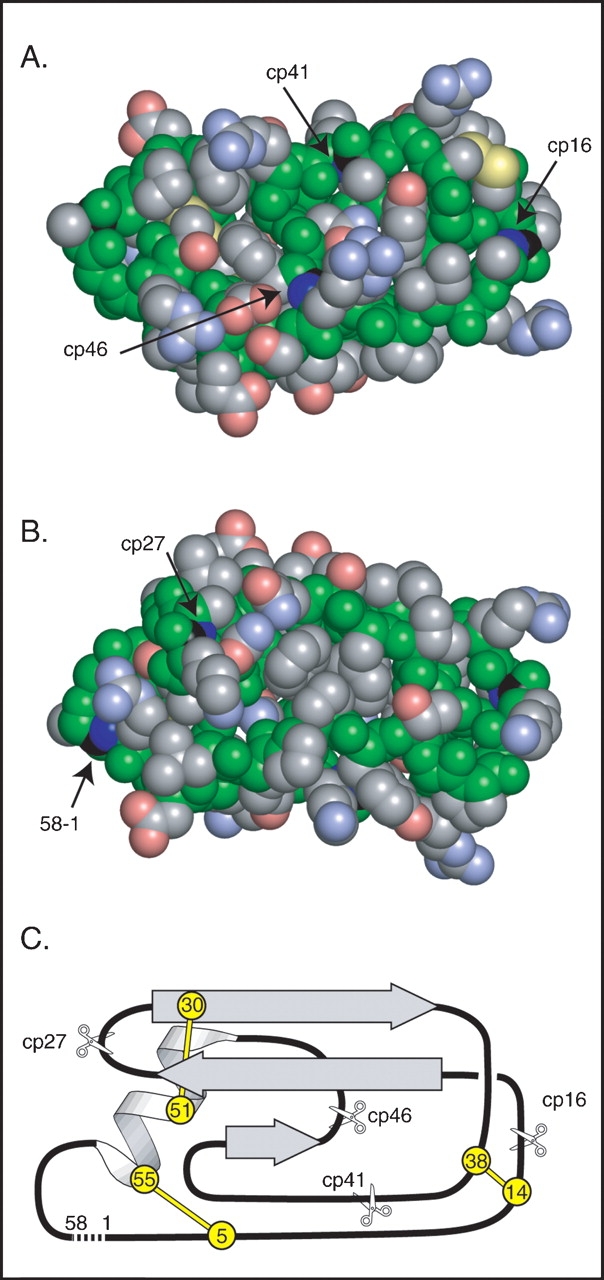
Design of circularly permuted BPTI variants. (A) Space-filling representation of a model of circular BPTI indicating the sites of the natural N and C termini and the sites of cleavage in the circularly permuted proteins. Backbone atoms are colored green, and those of side chains are colored gray, blue, red, or yellow to indicate C, N, O, or S atoms, respectively. The C and N atoms of the peptide bonds broken to generate the termini in the natural and circularly permuted protons are colored black and dark blue. The model of circular BPTI was constructed as described in Materials and Methods. (B) Space-filling model, as in A, but rotated 180°C about the horizontal axis to reveal the sites of the termini in the natural protein and cp27 variant. (C) Schematic representation of the topology and location of secondary structure elements in native BPTI. The locations and residue numbers of the six disulfide bonded Cys residues are indicated by circles, and the sites of cleavage in the circularly permuted proteins are indicated with scissors. A broken line connects the N and C termini of the natural sequence. The orientation in C is roughly equivalent to that in B.
