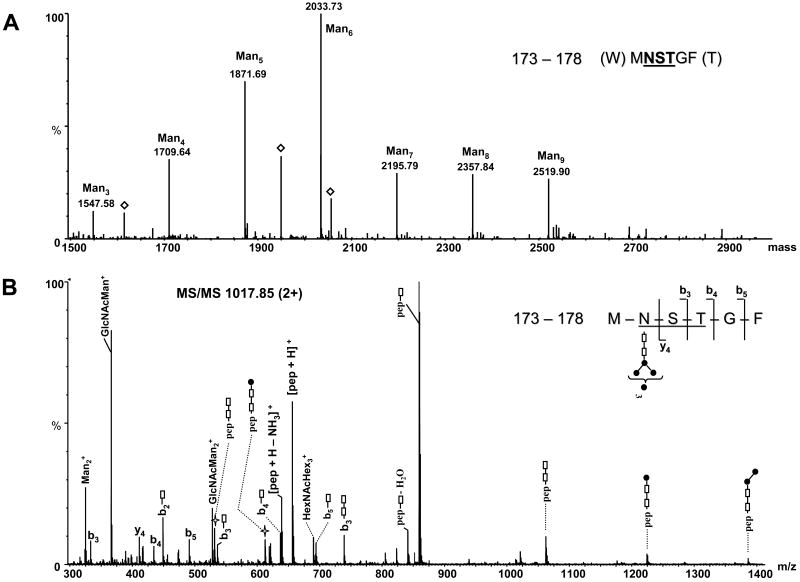
Figure 4.
ESI-MS of chymotryptic peptide 173–178: (A) Deconvoluted mass spectrum over the mass range 1500–3000, showing the glycopeptides with the amino acid sequence 173–178 containing high mannose glycans with composition from Man3 to Man9. The empty diamonds represent unrelated ions. (B) MS/MS of the precursor ion m/z 1017.85 (2+) corresponding to the chymotryptic peptide 173–178 containing the Man6 glycan at the glycosylation site N174 (N556). The glycopeptide and the structure of glycan are indicated at the top (right). The presence of the backbone b and y ions confirms the peptide sequence. Filled circles represent Man residues; empty rectangles represent GlcNAc. The number in the bracket represents maximum of Man residues attached to the trimannosyl chitobiose core. The majority of the observed ions are singly charged and the empty star denotes doubly charged ions.