Figure 1.
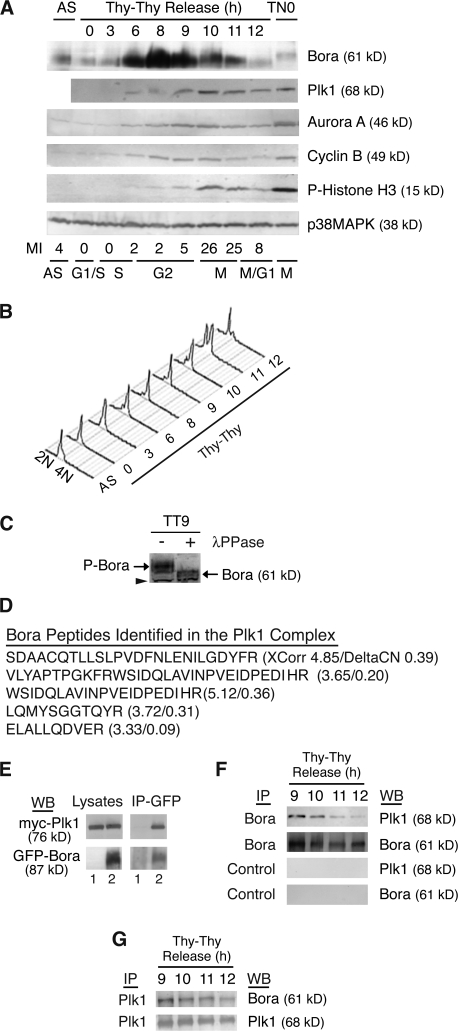
Bora is a cell cycle protein interacting with Plk1. (A and B) The levels of Bora fluctuate in the cell cycle. HeLa S3 cells were synchronized at the G1/S boundary by a double thymidine arrest (Thy–Thy), released into fresh media, and harvested at the indicated times. Protein levels were analyzed by Western blotting (A) and cell cycle profile was assayed by FACS with a propidium iodine staining and with an anti-MPM2 antibody staining (A and B). The MPM2 antibody recognizes mitotic phosphoproteins and was used here to determine mitotic index. p38MAPK served as a loading control. AS, unsynchronized cells; TN0, prometaphase cells synchronized by a thymidine-nocodazole arrest. (C) Phosphorylation of Bora in the cell cycle. G2 cells were collected at 9 h (TT9) after release from a double thymidine arrest and cell lysates were incubated with or without λ-phosphatase (λ-PPase). In a Western blot analysis, the phosphorylation state of Bora was determined by its mobility in SDS-PAGE. Arrowhead points to a cross reacting band, which also serves as a loading control. P-Bora, phospho-Bora. (D) Identification of Bora as a Plk1-interacting protein. Listed are peptides of Bora identified by mass spectrometry in the GFP-S-Plk1 complexes together with their XCorr and DeltaCN scores. (E) Ectopically expressed Bora interacts with Plk1. Myc-Plk1 was cotransfected with GFP (lane 1) or GFP-Bora (Lane 2) into HeLa cells and cells were harvested at 48 h after transfection. Cell lysates and the anti-GFP immunoprecipitates (IP) were assayed by Western blotting (WB). (F and G) Endogenous Bora interacts with Plk1 during Bora degradation. HeLa S3 cells were synchronized as in A. Endogenous Bora (F) or Plk1 (G) was immunoprecipitated with respective antibodies and associated proteins were analyzed by Western blotting. Control IP in F corresponded to immunoprecipitation with nonspecific IgG.