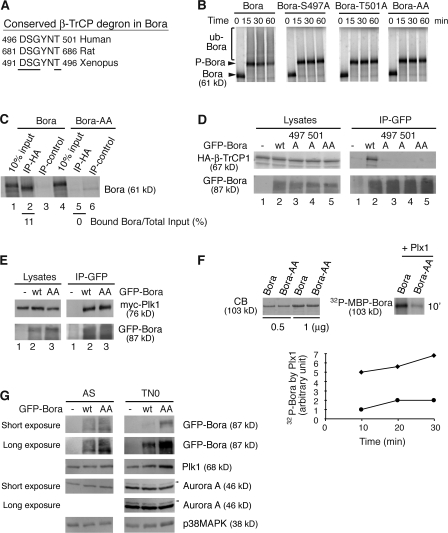
Figure 5.
The DSGxxT degron is required for Bora degradation. (A) The conserved β-TrCP degron in Bora from different species. Residues important for recognition by β-TrCP are underlined. (B) The β-TrCP degron is required for ubiquitination of Bora. In vitro ubiquitination assays were performed in TN0 extracts as described in Fig. 3 A. Bora-AA has both S497 and T501 mutated to A. (C) In vitro binding of HA-β-TrCP1 to Bora and Bora-AA were performed in nondepleted TN0 extracts, as described in Fig. 4 D. Numbers below lanes 2 and 5 represent β-TrCP1–bound Bora relative to their respective total inputs. (D and E) The β-TrCP degron is required for the association of Bora with β-TrCP1 but not for the association of Bora with Plk1 in vivo. HA-β-TrCP1 (D) or myc-Plk1 (E) was cotransfected into HeLa cells with either GFP (lane 1) or GFP-Bora (wild type or mutants) and cells were harvested 48 h after transfection. Cell lysates and the anti-GFP immunoprecipitates were assayed by Western blotting. (F) Plk1 phosphorylates the Bora degron. Recombinant MBP-Bora and MBP-Bora-AA were phosphorylated by purified recombinant Plx1 (X. laevis homologue of Plk1) in the presence of radioactive ATP. The kinetics of Plx1-mediated phosphorylation was quantified and plotted. The amounts of MBP-Bora and MBP-Bora-AA were determined by Coomassie blue staining (CB). ♦, Bora; •, Bora-AA. (G) The DSGxxT degron is recognized in vivo. HeLa cells were transfected with GFP, GFP-Bora, or GFP-Bora-AA and harvested as asynchronous culture (AS) at 34 h after transfection or as prometaphase cells (TN0) at 48 h after transfection after a thymidine-nocodazole arrest. Total cell lysates were analyzed by Western blotting. Arrowheads point to the active and phosphorylated Aurora A (Hirota et al., 2003).