Abstract
Sugar binding studies, inactivation, unfolding, and refolding of native Jacalin (nJacalin) from Artocarpus integrifolia and recombinant single-chain Jacalin (rJacalin) expressed in Escherichia coli were studied by intrinsic fluorescence and thermal and chemical denaturation approaches. Interestingly, rJacalin does not undergo any proteolytic processing in an E. coli environment. It has 100fold less affinity for methyl-α-galactose (Ka: 2.48 × 102) in comparison to nJacalin (Ka: 1.58 × 104), and it also binds Thomsen-Friedenreich (TF) disaccharide (Galβ1–3GalNAc) with less affinity. Overall sugar binding characteristics of rJacalin are qualitatively similar to that of nJacalin (Gal<MeαGal<MeαTFdisaccharide). Circular dichroism studies at near- and far-UV, thermal, and chemical denaturation studies reveal that the rJacalin behaves like nJacalin. Guanidine hydrochloride-induced denaturation, followed by renaturation, yielded total recovery of sugar binding activity of rJacalin in comparison to partial recovery for nJacalin. This signifies the minor changes in the refolding pathways between native and recombinant lectins. The stability of rJacalin is dramatically reduced in the extreme pH range unlike nJacalin. Both lectins do not bind 1-anilino-8-naphthalene sulfonic acid (ANS) in the pH range of 5 to 12 but they do in the pH range of 1–3. Solute quenching studies of the lectin using acrylamide, KI, and CsCl indicated that the tryptophan residues have full accessibility to the neutral quencher and poor accessibility to ionic quenchers. In summary, biophysical and biochemical studies on the native versus recombinant Jacalin suggest that post-translational modification, i.e., the processing of Jacalin into two chains is probably not a prerequisite for sugar binding but may be required for higher affinity.
Keywords: native Jacalin, agglutination activity, recombinant single chain Jacalin, sugar specificity, Thomsen-Friedenreich antigen, fluorescence studies, thermal unfolding, refolding
Plant lectins, since their discovery, have evoked enormous interest in terms of understanding their carbohydrate specificity and structure to exploit them for various biological applications. Lectins can be broadly classified into several groups based on their carbohydrate specificity toward glucose, galactose, mannose, high mannose, etc (Sharon and Lis 2001). Several lectins exhibit specificity toward blood-group saccharides and hence their immediate application was to identify and distinguish various blood groups.
Among the galactose-specific lectins, the lectin from Artocarpus integrifolia, known in the literature as Jacalin (native Jacalin or nJacalin), has attracted much attention since its isolation and characterization (Bunn-Moreno and Campos-Neto 1981; Roque-Barreira and Campos-Neto 1985; Sastry and Surolia 1986; Sastry et al. 1986; Mahanta et al. 1990, 1992; Kabir 1998). Jacalin is a 65–66-kDa, tetrameric protein, which exhibits specificity toward galactose and its α-linked derivatives. Among many other interesting properties, the lectin exhibits specificity toward human IgA, especially to IgA1 subclass (Roque-Barreira and Campos-Neto 1985). Jacalin also exhibits specificity toward tumor-specific disaccharide viz. Thomsen-Friedenreich (TF) disaccharide, i.e., Galβ1→3GalNAcαSer/Thr (Sastry et al. 1986; Mahanta et al. 1990, 1992). Another interesting property of Jacalin is revealed in the research of acquired immuno deficiency syndrome (AIDS) because Jacalin specifically stimulates CD4+ cells in comparison to primary T cells (Pineau et al. 1989, 1990; Favero et al. 1993; Corbeau et al. 1994; Lafont et al. 1994, 1996; Blasco et al. 1995; Tamma et al. 1996). It has been shown that Jacalin binds to porphyrin rings with high affinity and could be used in photodynamic therapy (Dougherty et al. 1978; Levy 1995; Komath et al. 2000).
Considering the potential applications of Jacalin, we have aimed to investigate the folding, function, and carbohydrate specificity of Jacalin at molecular level. Although, the native protein can be used to delineate some of the properties, understanding at the molecular level is greatly hindered due to the inability to modify the backbone of native Jacalin. The diversity in specificity of lectins or Jacalin per se, might have arisen due to various hidden factors such as multimeric, multichain, and partial glycosylation. It is not clear to date which property (i.e., multimeric form/multi chain/post translational modifications) contributes to the sugar specificity and/or the affinity they exhibit. It has been suggested by Vijayan and colleagues that the newly generated amino terminus (Gly residue) in the α-chain of Jacalin, post excision of “T-S-S-N” peptide, may contribute to higher affinity (Jeyaprakash et al. 2003). Recently, it was argued by Pierre Rouge and coworkers that Jacalin also binds mannose as revealed by surface plasmon resonance and X-ray crystallography but the affinity for mannose residues is several-fold lower in comparison to galactose and Me-α-Gal (Bourne et al. 2002; Houles et al. 2002).
Considering the importance in understanding the carbohydrate affinity/specificity, we chose Jacalin for detailed studies since it can serve as an excellent model system because of its smaller size and available knowledge on its 3D structure. Moreover, Jacalin has several other family members with different sugar specificity. In view of these advantages, we aimed to understand the role of multi-chain requirement in the carbohydrate specificity elicited by Jacalin by using a single-chain recombinant protein to understand the sugar binding properties, unfolding, and refolding of Jacalin in solution. Biophysical and biochemical studies presented herein provide an important insight on the role of multichain, multimeric, and glycosylation requirements of Jacalin for its sugar binding activity.
Results
In Artocarpus integrifolia, Jacalin is synthesized as a pre-pro-lectin, consisting of a 21-amino-acid signal sequence, 39-amino-acid pro-peptide, 20-amino-acid β-chain, a linker peptide “Thr-Ser-Ser-Asn,” and 133-amino-acid α-chain, which has the sugar binding site. In mature Jacalin, the signal sequence (21 aa) and pro-peptide (39 aa) are removed, probably through post- or co-translational processing as the mechanism is not yet clear. The four-amino-acid linker peptide “T-S-S-N,” which connects both β- and α-chains, is excised to generate two chains of Jacalin. In addition to the processing, Jacalin is probably mildly N-glycosylated, at Asn74 as interpreted by 1H-NMR data of nearby Thr76 resonance (Capon 1990). This is the most likely site of glycosylation as the other two potential sites at amino acid positions 16 and 35 were not glycosylated based on sequencing studies (Ruffet et al. 1992).
Molecular cloning and characterization of recombinant Jacalin
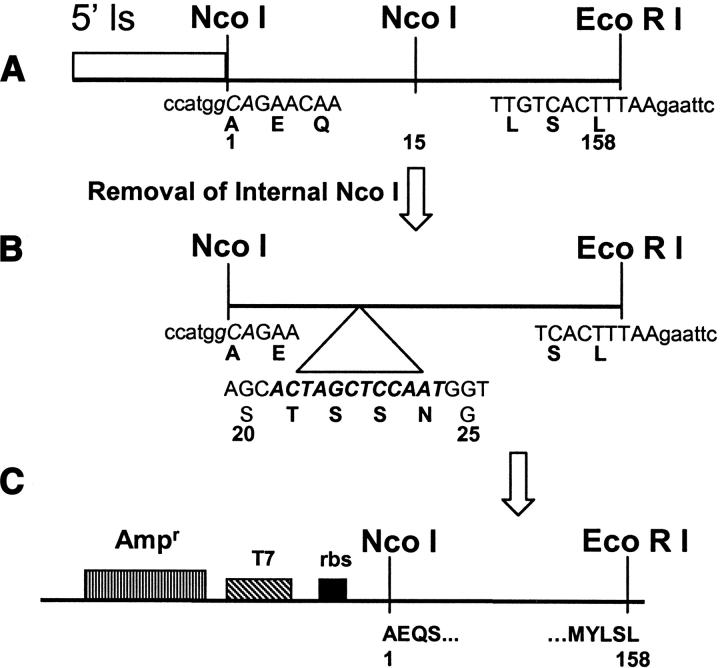
The cloning strategy employed to isolate the Jacalin gene has been depicted in Figure 1 ▶. In this strategy, we have removed the first 39 amino acids of Jacalin, which is said to be part of pro-Jacalin, i.e., this signal sequence has not been found in mature form of Jacalin (Yang and Czapla 1993). In order to obviate the bacterial expression problems due to N-end rule, we have cloned the Jacalin gene with Ala as the amino-terminal amino acid instead of Asn (Hirel et al. 1989; Tobias et al. 1991; Lathrop et al. 1992). The dideoxy nucleotide sequencing of several isolates has confirmed our cloning strategy, outlined in Figure 1 ▶. The isolate, pT7-rJac1, which is used for the studies described in this article, corresponds to the pSKc-JA3 clone of an earlier report (Yang and Czapla 1993). Moreover, amino-terminal sequencing by Edman degradation of the Escherichia coli-expressed rJacalin yielded the amino terminal amino acids “A-E-Q-S-G. . .,” which is in complete agreement with the nucleotide sequence deduced. The protein sequencing has also confirmed that the recombinant Jacalin does not undergo any proteolytic cleavage in the E. coli, as it always migrated as a homogenous, single band on SDS-PAGE as shown in Figure 2 ▶ (lane 1). In addition, we did not find any other amino-terminal amino acid during Edman degradation other than Ala (data not shown).
Figure 1.
Cloning of the gene of Jacalin in single chain. (A) From total RNA of Jackfruit seed, cDNA synthesis was carried out with oligo-dT as primer, followed by PCR amplification of Jacalin gene by vent DNA polymerase using an upstream gene-specific primer containing NcoI site and a downstream gene specific primer containing an EcoRI site. The ATG sequence present in the NcoI site (ccATGg) acts as the initiation codon for the translation of the mature Jacalin polypeptide. Temperature conditions used for PCR amplification were 1 min at 95°C for denaturation, 1 min at 60°C for annealing, and 3 min 30 sec at 72°C for extension. At the end of 25 cycles, an additional 10 min at 72°C was given for extension. (B) The above amplified gene product had one internal NcoI site at 15th amino acid position, which was removed by silent mutation through site directed mutagenesis using internal Nco I knockout primer. Unlike the native protein, α and β chain of rJacalin are linked with T-S-S-N loop as indicated in the figure, to make a single chain protein. (C) The above mutated PCR product was cloned between NcoI and EcoRI site in the pT7Nc vector described by Vandana et al. earlier (1997).
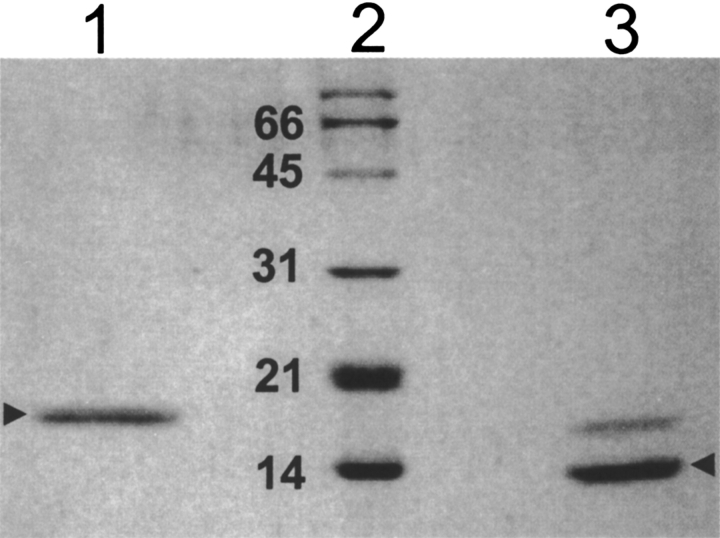
Figure 2.
SDS-PAGE of purified native as well as recombinant single chain Jacalin. The nJacalin was purified as described earlier (Sastry and Surolia 1986; Sastry et al. 1986; Mahanta et al. 1990, 1992) and the single chain rJacalin was purified as described under Materials and Methods and electrophoresed on 15% SDS-PAGE. (Lane 1) 2 μg of rJacalin, (lane 2) SDS-PAGE standard low-range marker (Bio-Rad). The molecular weights are written in kDa. (Lane 3) 2 μg of purified nJacalin.
Molecular mass and subunit structure
The molecular mass was estimated by size-exclusion chromatography on a Sephacryl-S300 HR column using an FPLC. The recombinant Jacalin co-eluted with nJacalin with a molecular mass of 66 kDa. These data, in conjunction with the SDS-PAGE migration pattern, indicate that the recombinant Jacalin exists as a tetramer as it also exhibited hemagglutination which can be inhibited by Me-α-Gal (data not shown).
Circular-dichroism studies
The rJacalin showed a typical far-UV CD spectrum observed for proteins with high proportion of β-sheet content, with minimum at 218 nm (Fig. 3 ▶). The relative percentage of structural elements calculated using CD-Pro software package for analyzing protein CD spectra was in complete agreement with earlier reports as well as the crystal structure. The near-UV CD spectrum of Jacalin is also in complete agreement with the data shown by Mahanta et al. (1990). It is interesting to note that the recombinant Jacalin exhibits an identical secondary-structure pattern in comparison to native Jacalin. From these observations, it is reasonable to conclude that the recombinant Jacalin is nearly identical to that of native Jacalin as far as the secondary structure is concerned.
Figure 3.
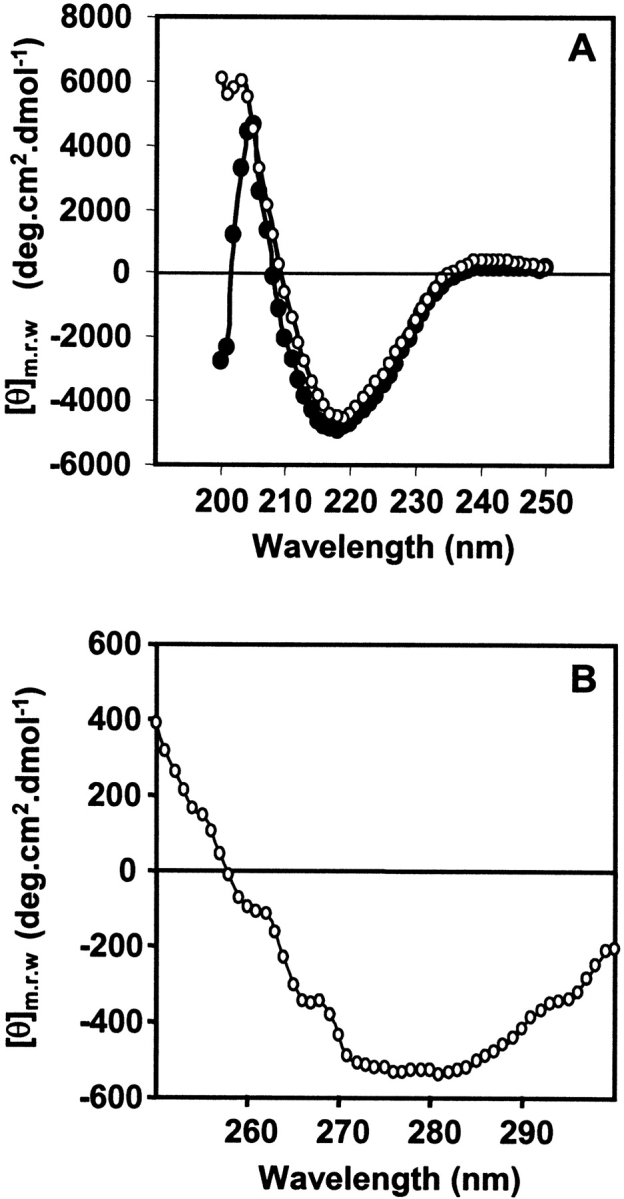
Circular dichroism spectra of native and recombinant Jacalin. (A) The far-UV CD spectra of single chain rJacalin (•). (B) Near-UV CD spectra of single chain rJacalin (○). Where [θ] denotes mean residue ellipticity. All spectra were obtained after an average of 10 scans each.
Sugar binding studies
The fluorescence enhancement of nJacalin, after addition of Me-α-Gal, was approximately 35% while that of rJacalin was approximately 8%. These changes were exploited to obtain the association constants for the binding of various sugars to nJacalin and rJacalin as reported earlier (Sastry and Surolia 1986; Gaikwad et al. 1998 Gaikwad et al. 2002; Gaikwad and Khan 2003). A dramatic difference between nJacalin and rJacalin is the affinity for mono and disaccharides. The rJacalin has 100-fold less affinity of binding Me-α-Gal (Ka = 2.48 × 102 ± 0.24 × 101 and Galβ(1→3)GalNAc-α-Me (Me-α-TF antigen: Ka = 5.37 × 102 ± 0.21 × 102) than nJacalin (Ka for Me-α-Gal = 1.58 × 104 ± 0.31 × 103 and Me-α-TF antigen Ka = 4.00 × 105 ± 0.16 × 105). The rJacalin showed very little signal change in the fluorescence emission when titrated with Gal, GalNAc, Mannose and Galβ1→3GalNAc (Table 1). However, Gal, GalNAc and Galβ1→3GalNAc but not Mannose or Me-α-Man, inhibited the hemagglutination by rJacalin.
Table 1.
Association constants of select sugars for their binding to nJacalin and rJacalin based on changes in the intrinsic fluorescence emission
| Association constants (Ka) | |||
| S number | Sugar | N-Jac | R-Jac |
| 1 | Galactose | 1.20 × 103 (±0.64 × 102) | NS |
| 2 | Me-α-Gal | 1.58 × 104 (±0.31 × 103) | 2.48 × 102 (±0.24 × 101) |
| 3 | GalNAc | 1.90 × 103 (±0.95 × 102) | NS |
| 4 | Me-α-GalNAc | 9.30 × 104 (±0.18 × 104) | 2.51 × 102 (±0.12 × 102) |
| 5 | Galβ(1–3)GalNAc | 4.00 × 104 (±0.16 × 104) | NS |
| 6 | Galβ(1–3)GalNAcαMe | 4.00 × 105 (±0.24 × 105) | 5.37 × 102 (±0.21 × 102) |
| 7 | Me-β-Galactose | 2.00 × 102 (±0.05 × 102) | NB |
| 8 | Mannose | NB | NB |
| 9 | Me-α-Mannose | NB | NB |
| 10 | Mellibiosea | 6.25 × 103 | 0.422 × 102 |
The association constants were obtained as described in Materials and Methods for nJacalin and rJacalin and were calculated as per the published literature (Sastry and Surolia 1986; Sastry et al. 1986; Mahanta et al. 1990, 1992; Gaikwad and Khan 2003). The data are an average of three independent measurements and the numbers in parentheses are standard deviations. NS indicates no change in intrinsic fluorescence signal was observed but inhibits hemagglutination activity of rJacalin. NB indicates no change in intrinsic fluorescence signal as well as no inhibition of hemagglutination.
a Values obtained at 20°C taken from Sastry et al. 1986.
Denaturation studies
The fluorescence emission spectrum of the native as well as recombinant Jacalin showed an emission maximum at 334 nm, which characterizes nonpolar environment of the tryptophan residues. Upon denaturation, with increasing concentration of Gdn-HCl (1–6 M), both lectins showed a consistent decrease in the fluorescence intensity at 334 nm, with a concomitant increase in emission maxima at 356 nm. In essence, most of the tryptophans in the protein were being exposed to the solvent. The ratio of fluorescence intensities, F335/356, at 334 and 356 decreases from 1.41 to 1.27 for native Jacalin and 1.42 to 1.24 for recombinant single-chain Jacalin. The denaturation or the partial unfolding of the lectin is reflected into decrease in the F334/356 ratio.
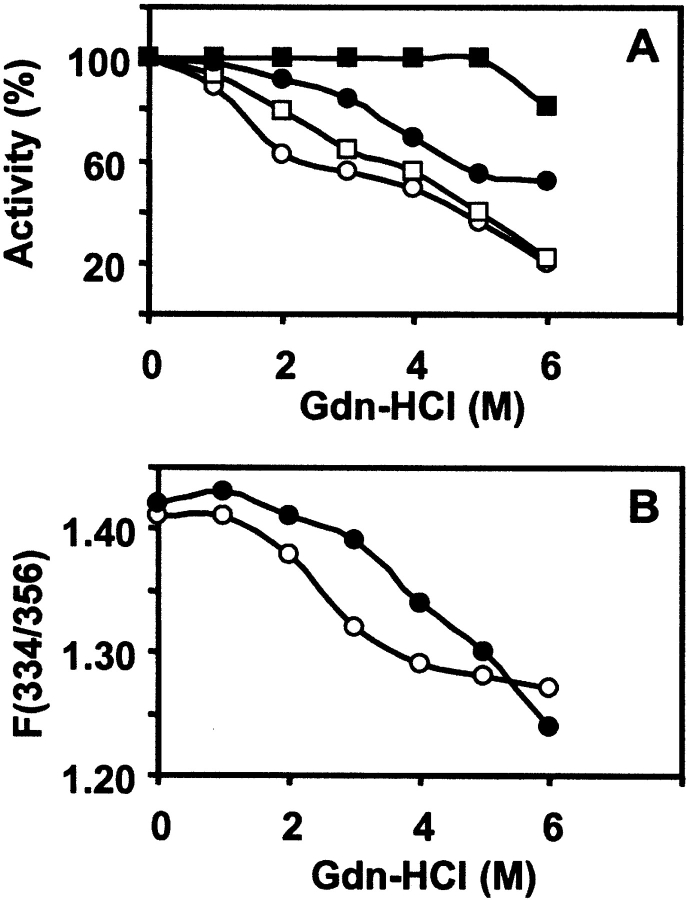
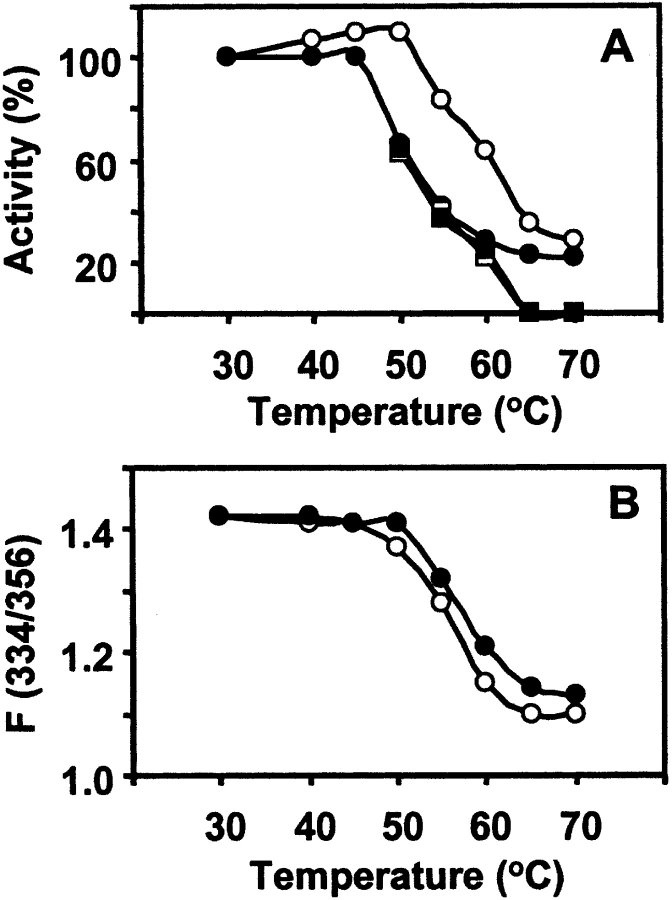
The maximum enhancement in the intrinsic fluorescence emission of lectin due to the binding of sugar, Me-α-Gal, was taken as 100% activity of the lectin, and the residual activity was calculated as described earlier for Artocarpus hirsuta lectin (Gaikwad et al. 2002; Gaikwad and Khan 2003). The inactivation of the lectin was proportional to the concentration of Gdn-HCl as seen in Figure 4 ▶, A and B. There was a significant decrease in percent activity of nJacalin as compared to rJacalin, which is relatively stable. The rJacalin shows a slow decrease in percentage activity as compared to nJacalin. It is evident from the above observation that nJacalin is more susceptible than rJacalin to Gdn-HCl mediated denaturation. A reasonable explanation for the observation could be that the nJacalin is a two-chain protein and its denaturation might have led to the separation of the α- and β-chains completely. As a result, recovery of sugar binding activity after renaturation is rather poor for nJacalin. In case of rJacalin, the α- and β-chains are connected by T-S-S-N linker peptide, hence, it exhibited a better recovery of sugar binding activity.
Figure 4.
Chemical unfolding and refolding studies. (A) Gdn-HCl induced denaturation and renaturation of nJacalin as well as rJacalin (0.6 μM) at 30°C monitored by the fluorescence of Trp at required Gdn-HCl concentration. Protein sample was incubated for 4 h prior to measurements. The fluorescence spectra were recorded between 300 nm and 400 nm with the excitation wavelength of 280 nm. The percent residual activity after incubation of protein at a given concentration of Gdn-HCl was determined as described in the Materials and Methods section. For renaturation, the samples were diluted 10 times in phosphate buffer and incubated for 1 h at 30°C, spectra were observed, and activity was assessed. The symbols used in this figure are nJacalin denaturation (○), nJacalin renaturation (□), rJacalin denaturation (•), rJacalin renaturation (▪). (B) Denaturation by Gdn-HCl, ratio F(334/356). The symbols used are nJacalin (○) and rJacalin (•).
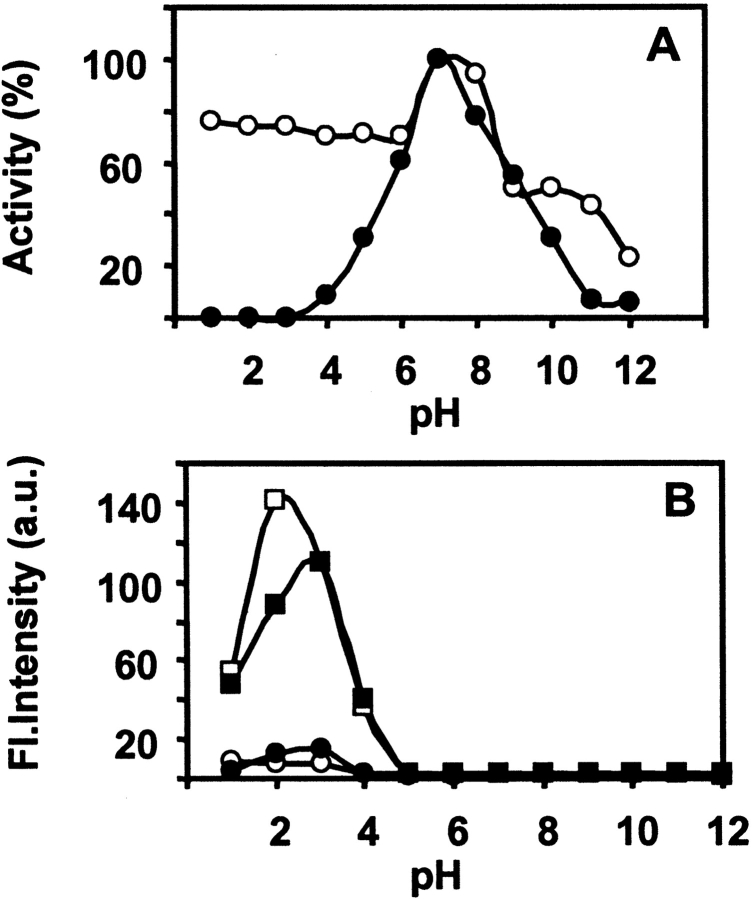
Effect of pH on both the lectins was studied in the pH range of 1–12. The percentage activity was measured in the same way, as described above. The nJacalin is quite stable at extreme acidic pH with little loss in activity up to 50°C (data not shown) in comparison to rJacalin, which is highly susceptible at extreme acidic pH even at 30°C (Fig. 5A ▶). This clearly indicates that significant differences exist between nJacalin and rJacalin. Both the proteins are, however, unstable at extreme alkaline pH. Thermal denaturation of both the lectins was measured as a function of increasing temperature from 30°C to 70°C. As seen from Figure 6 ▶, A and B, it can be observed that nJacalin is stable up to 50°C while rJacalin is stable up to 45°C only. Both the proteins lost their activity with further increase in temperature. It is not clear whether this could be due to the presence of mild glycosylation in nJacalin, which may offer some resistance to thermal denaturation. However, this observation needs to be deciphered further. In comparison, A. hirsuta lectin, nearly identical to nJacalin (Rao et al. 2004), also exhibited differential modes of renaturation after denaturation. While chemical denaturation yielded partial renaturation, thermal denaturation was irreversible (Gaikwad et al. 2002; Gaikwad and Khan 2003).
Figure 5.
The pH unfolding and refolding studies. (A) Effect of pH on the percent residual activity of nJacalin (○) as well as rJacalin (•) at 30°C. (B) Fluorescence emission spectra for ANS binding in the presence of nJacalin as well as rJacalin at 30°C and 55°C. The symbols used are nJacalin at 30°C (○), rJacalin at 30°C (•), nJacalin at 55°C (□), rJacalin at 55°C (▪).
Figure 6.
Thermal unfolding and refolding studies. (A) The percent residual activity of nJacalin as well as rJacalin after incubation of protein from 30°C to 70°C and for renaturation of nJacalin as well as rJacalin after recovery of sugar binding activity when duplicate samples were cooled gradually to 30°C. The symbols used are nJacalin denaturation (○), nJacalin renaturation (□), rJacalin denaturation (•), rJacalin renaturation (▪). (B) Denaturation by temperature, ratio F(334/356). The symbols used are nJacalin (○), rJacalin (•).
Refolding of protein
Renaturation/refolding of nJacalin and rJacalin was assessed with respect to the extent of recovery of sugar binding activity. The renaturation of the denatured (Gdn-HCl-induced) sample was carried out by diluting the samples 10 times with 50 mM phosphate buffer. The fluorescence emission spectra and sugar binding activity of the diluted samples were examined after 1 h. The rJacalin shows substantial reactivation in the 1-to-6-M Gdn-HCl, whereas the nJacalin showed only partial reactivation (Fig. 4A ▶). This is a significant difference between the nJacalin and rJacalin as they differ in two important aspects: (1) single chain versus two chains and (2) glycosylated versus unglycosylated proteins. In the case of renaturation after thermal denaturation, the protein sample was incubated for 15 min at indicated temperatures and then slowly brought to 30°C. From the percent residual activity data, it is clear that the thermal denaturation is an irreversible process for both lectins. There was no recovery of sugar binding activity for native as well as for recombinant Jacalin (Fig. 6A,B ▶).
Light-scattering studies
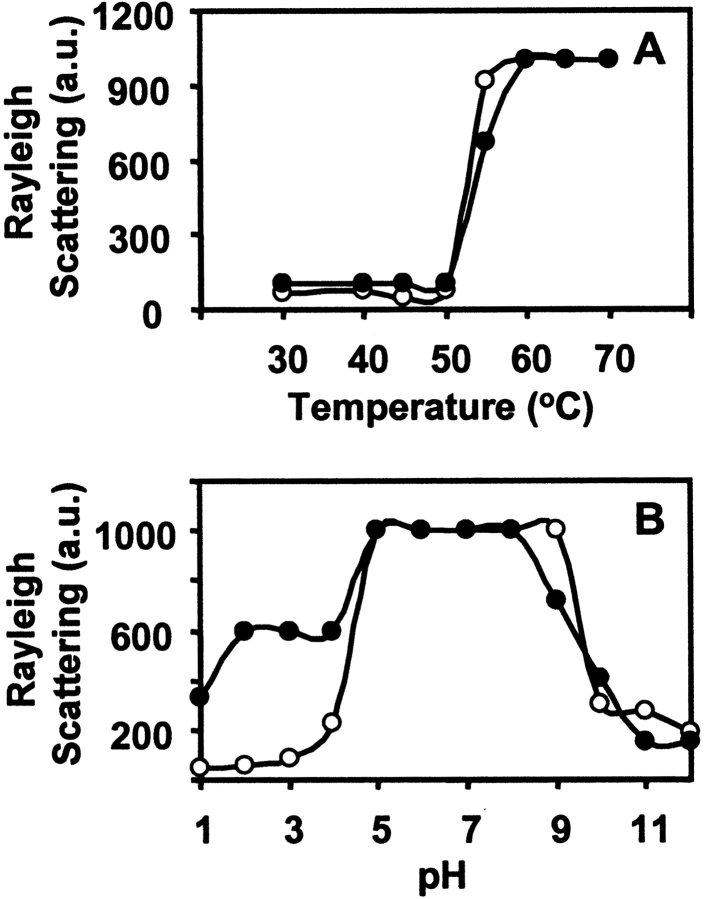
Both nJacalin and rJacalin aggregate above 50°C (Fig. 7A ▶), as indicated by the high light-scattering intensity. In case of thermal denaturation at 55°C at different pH, the aggregation of denatured nJacalin does not take place at extreme pH (acidic and basic), however, slight aggregation of rJacalin was observed at 55°C, at extreme acidic pH as seen in Figure 7B ▶. One likely reason could be that the extreme acidic pH may have resulted in charge repulsion, which does not allow lectins to aggregate. In the pH range of 5–9, high light-scattering was observed indicating that on thermal denaturation, both Jacalins were aggregated (Fig. 7B ▶).
Figure 7.
Comparison of Rayleigh light scattering of native and recombinant Jacalin to study the aggregation parameters of protein. (A) Effect of temperature on light scattering of nJacalin (○) and rJacalin (•) protein. (B) Effect of pH on light scattering of nJacalin (○) and rJacalin (•) lectin.
ANS-binding studies
Differences in surface hydrophobicity were studied by exploiting the interaction of 1-anilino-8-naphthalene sulfonic acid (ANS) to the lectins. Salient differences in surface hydrophobicity reflect the extent of the exposure of hydrophobic patches/side chains of the protein in general (Stryer 1965). Binding of ANS to the proteins occurs only upon the exposure of hydrophobic patches/clusters during the unfolding and refolding process (Semisotnov et al. 1991). The data in Figure 5B ▶ show the fluorescence emission of ANS in the presence of nJacalin and rJacalin at 30°C and 55°C. Both nJacalin and rJacalin show a little binding to ANS in the pH range of 1–3 at 30°C. The ANS binding increases with increase in temperature indicating the exposure of hydrophobic patches on the surface of protein on thermal denaturation at acidic pH. At 55°C, the ANS binding for both the lectins increases about 10-fold as compared to ANS binding at 30°C.
Solute quenching
Solute quenching of tryptophan fluorescence provides information on the microenvironment of the lectin near tryptophan residues. The intrinsic fluorescence of native as well as rJacalin was studied using dynamic quenchers (acrylamide, CsCl, and KI). Acrylamide is a neutral quencher with ability to penetrate into the hydrophobic cores of proteins and can discriminate the degree of exposure of tryptophan residues in the proteins. The intrinsic fluorescence of nJacalin as well as rJacalin was 100% quenched by acrylamide, indicating that there was almost no steric shielding of tryptophan residues by neighboring protein segments in both proteins.
Ionic quenchers, being charged and heavily hydrated, are anticipated to quench only surface tryptophan residues, if any. The data in Table 2 show that the CsCl caused only 5% and 0% quenching of fluorescence of nJacalin and rJacalin, respectively. Interestingly, KI caused only 8% and 0% quenching of fluorescence of rJacalin and nJacalin, respectively. This indicates that most of the tryptophan residues in both the proteins were buried inside the core of molecule and not accessible to the ionic quenchers. However, the quenching of nJacalin fluorescence by CsCl, and quenching of rJacalin fluorescence by KI and their failure to quench the complementary lectins, clearly indicates that the micro-environment of the tryptophan residues are different in nJacalin and rJacalin, the former being electronegative and the latter electropositive.
Table 2.
Quenching parameters for nJacalin and rJacalin lectin
| rJacalin | nJacalin | |||||
| Quencher | Kq | Fa (%) | Quench (%) | Kq | Fa (%) | Quench (%) |
| Acrylamide | 3.49 | 100 | 100 | 3.66 | 100 | 100 |
| Kl | 21 | 0.082 | 8 | ND | ND | ND |
| CsCl | ND | ND | ND | 29 | 0.05 | 5 |
The quenching measurements were obtained as described in Materials and Methods for nJacalin and rJacalin as well as by following the approaches published earlier (Chipman et al. 1967; Gaikwad et al. 1998). The data are an average of three independent measurements. ND indicates quenching not detectable.
Discussion
The unfolding and refolding properties of both the lectins were studied by chemical and thermal denaturation (Fig. 4 ▶–6 ▶). In the present studies, it was observed that the nJacalin does not aggregate at extreme acidic as well as alkaline pH, while rJacalin shows slight aggregation at extreme acidic pH. High light scattering was observed in the pH range of 5–9 for both the lectins. In this pH range, the exposed hydrophobic patches due to thermal unfolding could be interacting with each other leading to aggregation due to hydrophobic interaction. These hydrophobic interactions would not take place in the extreme acidic and alkaline pH due to charge repulsion and therefore no aggregation should take place as observed for rJacalin and nJacalin (Fig. 7A ▶). The increase in light scattering intensity for both nJacalin and rJacalin with increase in temperature clearly indicates that the exposed hydrophobic patches are leading to aggregation of both the lectins (Fig. 7B ▶) due to hydrophobic interactions in the pH range of 5–9. This aggregation could be the reason why no ANS binding was observed in this pH range upon thermal denaturation. The loss of ANS binding above pH 9.0 could be due to loss of hydrophobic patches on complete denaturation. Similar results were also observed for A. hirsuta lectin (Gaikwad et al. 2002; Gaikwad and Khan 2003). The microenvironment of tryptophan residues, elucidated by quenching studies, indicate that most of the tryptophan residues are probably buried inside the core region of the protein and are only accessible to neutral quencher in both nJacalin and rJacalin (Table 2).
Among the monosaccharides, Me-α-Gal and among disaccharides, the α-isomer of TF-disaccharide, bound to nJacalin with high affinity. The association constants shown in Table 1 reveal that the sugar binding characteristics of rJacalin are qualitatively similar to that of nJacalin but the former has reduced affinity for the sugars used in the experiments. Noticeable feature is that no change in the intrinsic fluorescence emission signal was observed in case of sugars that do not have an α-substitution (Methyl or sugar) at their reducing end anomeric hydroxyl group. However, these saccharides inhibited the hemagglutination activity of rJacalin indicating that change in fluorescence intensity is probably due to the interaction of α-methyl (sugar) group with the aromatic residues in the binding site. In comparison, we could not observe any inhibition of hemagglutination activity of rJacalin by glucose, mannose, and their Me-α-derivatives. It has been observed in the crystal structure of Jacalin that the galactose and GalNAc of TF disaccharide occupy the primary site, the Me-α substitutions occupy the secondary site ‘A’. It has also been noted that while accommodating the Me-α group, Tyr122 residue undergoes movement to create space or groove (please refer to Table 2 and Figs. 3 ▶ and 5 ▶[b] in Jeyaprakash et al. 2003). In addition, the rJacalin did not display any preference for Me-β-derivatives of galactose very much like the nJacalin. Based on these observations we believe that the sugar binding pattern (Gal<MeαGal<MeαTFdisaccharide) observed for the rJacalin is not at random and the sugar binding traits of rJacalin are qualitatively similar to that of nJacalin. However, the major difference is the magnitude of affinity. Another interesting observation is that the rJacalin also bound to Me-α-TF-disccharide and with higher affinity as compared to Me-α-galactose (Table 1).
The binding of mono-saccharides to Jacalin occurs through a network of hydrogen bonds in which the O3, O4, and O6 of galactose make contacts with Gly1, Tyr122, Trp123 in addition to the side chain of Asp125 (Kabir 1998; Jeyaprakash et al. 2002, 2003). The O4 hydroxyl group of galactose forms hydrogen bonds with the side chain of Asp125. The Gly1 amino terminus is generated after the excision of “T-S-S-N” tetrapeptide connecting the β- and α-chains. Recently, it has been argued by Pierre Rouge and coworkers that the Jacalin also has affinity for mannose and its derivatives (branched) as observed by the binding of Jacalin to several glycoproteins (Arcelin-1, orosomucoid or a1-acid glycoproteins, ovomucoid, Soyabean agglutinin, native and desialylated human serotransferrin, native and desialylated calf fetuin, etc.) (Bourne et al. 2002; Houles et al. 2002). The affinity for mannose and its derivatives was very much lower in comparison to Me-α-Gal or Me-α-GalNAc or TF disaccharide. Based on the homology between the structural fold of Jacalin and Jacalin related proteins, i.e., β-prism pattern, it has been suggested by Pierre Rouge and coworkers that the proteolytic cleavage of Jacalin is perhaps crucial to the broad specificity it elicits. In other words, the proteolytic cleavage, post excision of T-S-S-N results in an “extended” binding site for Jacalin. As a result, it was argued that the Jacalin and MPL (another Jacalin-like lectin from Maclura pomifera) do not possess “monosaccharide-binding specificity.” However, our data unambiguously demonstrate that the rJacalin, with its linker sequence T-S-S-N intact, bound only to Galactose and its α-linked derivatives much better than mannose, very much like nJacalin. This observation clearly suggests that the binding of monosaccharides to Jacalin is not entirely dependent on the generation of a new amino terminus at Gly1 of the α-chain, post excision of T-S-S-N tetrapeptide. However, it may be required for higher affinity as suggested by Vijayan and colleagues (Jeyaprakash et al. 2003). We have prepared the rJacalin from an isolated, single colony, after transformation of E. coli with a well-characterized plasmid. Moreover, several batches of rJacalin preparations have yielded identical results. This is the first ever observation regarding the role of T-S-S-N in the binding site of Jacalin. These extensive controls raise an interesting question as to how Jacalin, without processing, recognizes galactose over mannose or glucose? It is possible that the specificity toward galactose is provided by the side chain of Asn of the tetrapeptide (T-S-S-N), which may substitute for the amino terminus of Gly1 (in case excision does not take place).
Regarding the decreased affinity of rJacalin for mono-and disaccharides, it is possible that the side chains of the tetra peptide T-S-S-N may cause steric hindrance during on or off reactions and hence, result in decreased affinity. It is relevant to mention here that the lectin Heltuba, which has similar structure to that of Jacalin, is a single-chain lectin in which the β-chain is covalently linked to the α-chain (Bourne et al. 1999). However, this lectin shows high specificity toward mannose instead of galactose, which could be a result of substitution of aromatic residues by nonaromatic residues in case of Heltuba and Artocarpin compared to Jacalin. In view of the resemblance of the three-dimensional structures of Jacalin and Heltuba, it was anticipated that the rJacalin may bind mannose and its derivatives, albeit weakly. To our surprise, we could not detect any measurable binding of rJacalin to mannose-coupled Sepharose or its derivatives despite working at high concentration. This observation clearly highlights that the aromatic residues or the microenvironment created by them may play a crucial role in orienting/dictating the carbohydrate specificity of Jacalin.
In summary, the rJacalin is anticipated to be an excellent model system for the study of galactose-binding nJacalins to understand its stability and overall folding. While X-ray crystallographic studies on Jacalin have yielded its frozen picture, the in-solution studies presented here have shown the function of the Jacalin in a dynamic environment. For the first time, our data have shed significant light on the carbohydrate-binding of Jacalin in the absence of excision of T-S-S-N peptide. The rJacalin has the same traits as nJacalin, however, its affinity for carbohydrate is much lower. Interestingly, the two-chain nJacalin does not regain its carbohydrate binding activity totally after denaturation with chaotropic agents like guanidine hydrochloride. However, the single-chain rJacalin regains its activity on removal of the agent. In addition, rJacalin is unstable at acidic pH unlike nJacalin. These observations raise another interesting question as to what factors then dictate the affinity of Jacalin toward carbohydrates. We believe that this as well as several related questions could be answered by taking up select point mutations of Jacalin and examine their crystal structures. For all such studies, the recombinant Jacalin presented here would be a very valuable tool. Currently such studies are underway in our laboratory.
Materials and methods
Me-α-Gal, Me-β-Gal, Galβ(1→3)GalNAc, Galβ(1→3)GalNAc-α-Me, and other sugars employed were obtained from Sigma Chemical Co. Cross-linked gaur gum was prepared as reported earlier (Sastry and Surolia 1986; Sastry et al. 1986). Guanidine hydrochloride (Gdn-HCl) and 1-anilino-8-naphthalene sulfonic acid (ANS) were purchased from Sigma Chemical Co. All other chemicals were of the highest purity available. Protein estimations were carried out by Bradford protein estimation kit from Bio-Rad. Protein-sequencing grade Immobilon Problot membranes were obtained from Millipore.
Buffers
Stock of 8 M Gdn-HCl (pH 7.0) was freshly prepared and filtered through 0.45 μm filter. Buffers used were (100 mM): Glycine-HCl for pH 1–3, Na-Acetate for pH 4.0, Citrate-Phosphate for pH 5.0 and 6.0, Phosphate for pH 7.0, Tris-HCl for pH 8.0 and 9.0, and Glycine-NaOH for pH 10–12.
Purification of A. integrifolia lectin
Native Jacalin (A. integrifolia) lectin from jackfruit seeds was purified as described earlier (Sastry and Surolia 1986; Sastry et al. 1986).
Cloning of single chain recombinant Jacalin
Total RNA was isolated, as reported earlier, from immature jack-fruit seeds, procured locally (Yang and Czapla 1993). The cDNA synthesis was carried out with oligo-dT as the primer with the help of SuperScript Reverse Transcriptase containing cDNA synthesis kit as per the instructions of the manufacturer (Life Technologies). The RT-PCR primers contained an NcoI and EcoRI (lowercase) in the upper (5′-GGAAGCGAccatggCAGAACAAAGCG3′) and lower (5′CGAGCGTTgaattcTTAAAGTGACAAGTAC-3′) primers, respectively. The cloning of Jacalin is schematically shown in Figure 1 ▶. The cDNA was denatured at 94°C for 2 min, followed by 30 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 3.5 min. An additional incubation at 72°C for 10 min was implemented to complete any unfinished strands. The amplified product, ~0.5-Kbp DNA fragment was cloned in pGEM3zf (–) vector (Promega Corp.). Since the Jacalin gene has internal NcoI and Nde I sites, the internal NcoI site was removed by site-directed mutagenesis in the same vector by established procedures. After mutagenesis, the resultant Jacalin clone is finally inserted into T7 promoter containing vector developed in-house as reported earlier (Vandana et al. 1997). The recombinant pT7-rJacalin was confirmed by dideoxy nucleotide sequencing using T7, SP6, and Jacalin specific internal primers.
Purification of recombinant Jacalin lectin
The recombinant plasmid pT7-rJac was transformed in both JM109 (DE3) or BL21 (DE3) expression hosts as described earlier (Vandana et al. 1997). Briefly, single colony from freshly transformed cells were inoculated into 10 ml LB medium with ampicillin (100 μg/ml) and the culture was grown at 37°C till the absorbance at λ600 reached 0.25–0.35 (about 8–10 h). The cells were collected by centrifugation, washed once with LB amp. One milliliter (1 ml) of this primary culture was inoculated into 1 L of LB amp and the culture was grown at 28°C until absorbance at λ600 reached 0.5 and the culture was induced with 1 mM isopropylthiogalactoside (final concentration). The cells from the expression culture were collected by centrifugation at 4000g for 10 min. The harvested cells were suspended in 10 ml lysis buffer (10 mM Tris-HCl [pH 8.0], 5 mM EDTA, and 200 mM NaCl) and were disrupted by sonication for 10 min at 40 duty cycles. The cell lysate was clarified by centrifugation at 16,000g for 40 min. The soluble fraction and pellet were stored at −70°C, or immediately processed as described below.
This soluble fraction was dialyzed against 10 mM acetate buffer (pH 5.0). The dialysate was centrifuged at 16,000g for 30 min to remove the precipitate. The supernatant was filtered with 0.22 μm filter to remove remaining particulate matter and loaded on SP-sepharose (Amersham Pharmacia) column pre-equilibrated with 10 mM acetate buffer (pH 5.0) and eluted with a step gradient of NaCl. The fractions positive for hemagglutination activity were pooled and further purified using a Mono-S column using an FPLC. The Mono-S bound rJacalin was eluted with 150–200 mM NaCl gradient and the protein was > 98% pure at this stage. We have routinely obtained about 100 μg of active lectin per liter of E. coli culture. The protein thus purified from the Mono-S column was extensively dialyzed against PBS.
N-terminal sequence analysis
The N-terminal sequence of the purified rJacalin lectin was analyzed by Applied Bio-systems using gas phase sequencing, after the lectin was electrophoretically transferred to Pro-Blot Immobilon membranes. We have obtained 10 cycles of the rJacalin sequence with high repetitive yields.
Circular-dichroism studies
Far-UV CD (200–250 nm) and near-UV CD (250–300 nm) spectra of both nJacalin and rJacalin (1 mg/ml) were recorded on a Jasco J715 spectropolarimeter in a 1-mm path length cell equipped with a circulating water bath. The spectra were collected with response time of 4 sec, and scan speed of 100 nm/s−1. Each data point was an average of ten accumulations.
Molecular mass and subunit structure
The molecular mass and subunit structure of purified rJacalin were determined by gel filtration through Sephacryl S-300 column (Amersham Pharmacia) using an FPLC system and by SDS-PAGE performed on samples with and without heating for 5 min in boiling water and in the presence or absence of 2-mercaptoethanol.
Hemagglutination assays
The hemagglutination activity of the lectins was determined by a twofold serial dilution using 2% rabbit erythrocytes in PBS (v/v). The hemagglutination titer was defined as the reciprocal of the highest dilution exhibiting observable hemagglutination. Inhibition of agglutination by different saccharides were also assayed by serially diluting the solution of saccharide in the microtiter wells, followed by the addition of lectin, followed by the addition of erythrocyte suspension after 30 min. The lowest concentration of saccharide that visibly decreased the extent of agglutination was defined as the minimum inhibitory concentration.
Effect of guanidine hydrochloride (Gdn-HCl)
Protein samples (0.6 μM) were equilibrated at the desired Gdn-HCl concentration for 4 h at 30°C. Unfolding as a function of Gdn-HCl was monitored by intrinsic tryptophan fluorescence emission, taken in a 1-cm quartz cell and recorded in the 300–400-nm region, when excited at 280 nm in a Perkin-Elmer LS50B spectrofluorimeter with attached circulating water bath. Excitation and emission band passes of 5 nm were used. Activity of the sample was measured at the same time.
Determination of association constants (Ka)
Fluorescence measurements were made using Perkin-Elmer LS50B fluorimeter. The protein sample (0.6 μM) in PBS was excited at 280 nm, aliquots of nonfluorescent ligands were added and emission spectra were recorded above 300 nm. From the intercept of double reciprocal plot of F0/Fc−F0 versus 1/[C], where F0 and Fc are the fluorescence intensities of the free protein and protein bound to nonfluorescent galactose derivatives at concentration [C], F∞ the fluorescence intensity at infinite sugar concentration was obtained. When log [C] is plotted against log [Fc−F0/F∞−Fc] the intercept on the abscissa yielded the association constant (Ka) value for the lectin-sugar interaction (Chipman et al. 1967; Gaikwad et al. 1998).
Qualitative sugar binding studies
Qualitative sugar binding assays of both lectins was carried by monitoring the changes in the intrinsic fluorescence of lectins as reported earlier (Sastry and Surolia 1986; Gaikwad et al. 2002; Gaikwad and Khan 2003). Both nJacalin and rJacalin yielded an enhancement in their intrinsic fluorescence at 334 nm after addition of the non-fluorescent ligands. The percent of sugar binding activity at various pH or Gdn-HCl or temperature was obtained with Me-α-Gal (4 mM) at saturating concentration as reported earlier (Gaikwad et al. 2002; Gaikwad and Khan 2003). Increase in the fluorescence of the native and recombinant lectin at pH 7.0 and 30°C after adding Me-α-Gal was taken as 100% activity for both, respectively.
Effect of temperature and pH
To examine the thermostability, lectin solutions (0.6 μM in PBS) were incubated in duplicates for 15 min from 30°C to 70°C. One of the duplicates was used to record spectrum and sugar binding activity. Intrinsic fluorescence emission of the protein solution was examined as described above. The other sample was brought to 30°C, centrifuged to remove any particulate matter, spectra were recorded and activity was estimated. The pH stability of the lectins was determined using the respective buffers. The lectin solution (0.6 μM) was incubated for 4 h at room temperature, spectra were recorded and activity was measured.
ANS-binding studies
8-Anilino-1-naphthalene sulfonate (ANS) emission spectra were recorded in the range of 400–550 nm with excitation at 375 nm using slit widths of 5 nm. The change in the ANS fluorescence induced by the binding of the native as well as recombinant Jacalin was followed by recording the spectra at constant concentration of protein (0.6 μM) and ANS (50 μM), at different pH at 30°C and 55°C.
Light-scattering studies
Rayleigh light-scattering experiments were carried out with the spectrofluorimeter to follow protein aggregation during thermal denaturation at different temperature and different pH at 30°C and 55°C. The excitation and emission wavelength used were 400 nm. The excitation slit width was 5 nm and emission slit width was 2.5 nm. The scattering intensity was monitored for 180 sec.
Solute quenching
Titrations of rJacalin and nJacalin with acrylamide, CsCl, and KI were performed as described by Gaikwad et al. (1998). The iodide solution contained sodium thiosulphate (200 μM) to suppress tri-iodide formation. Defined amounts of the quencher (10 μl) were added from a stock of 5.0 M to a 2-ml (0.6 μM) protein solution. All quenching experiments were done in 50 mM phosphate buffer. The fluorescence spectra were recorded after 4 min and data were analyzed according to the following equations (Lehrer and Leavis 1978; Eftink and Ghiron 1981):
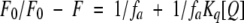 |
where F and F0 are the fluorescence intensities at 334 nm in the presence and absence of the quencher at concentration [Q], respectively. Kq is the modified Stern-Volmer quenching constant and fa is the fraction of tryptophan residues accessible to the quencher.
Acknowledgments
We thank Dr. G.C. Mishra for encouragement and Mr. Anil Lotke for technical assistance. A.S. is a senior research fellow of NCCS. The financial assistance was provided by the Department of Biotechnology, Government of India to K.S. and M.I.K.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.04968804.
References
- Blasco, E., Barra, A., Nicolas, M., Lecron, J.C., Wijdenes, J., and Preud’homme, J.L. 1995. Proliferative response of human CD4+ T lymphocytes stimulated by the lectin jacalin. Eur. J. Immunol. 25 2010–2018. [DOI] [PubMed] [Google Scholar]
- Bourne, Y., Zamboni, V., Barre, A., Peumans, W.J., van Damme, E.J., and Rouge, P. 1999. Helianthus tuberosus lectin reveals a widespread scaffold for mannose-binding lectins. Struct. Fold. Des. 7 1473–1482. [DOI] [PubMed] [Google Scholar]
- Bourne, Y., Astoul, C.H., Zamboni, V., Peumans, W.J., Menu-Bouaouiche, L., van Damme, E.J., Barre, A., and Rouge, P. 2002. Structural basis for the unusual carbohydrate-binding specificity of jacalin towards galactose and mannose. Biochem. J. 364 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn-Moreno, M.M. and Campos-Neto, A. 1981. Lectin(s) extracted from seeds of Artocarpus integrifolia (jackfruit): Potent and selective stimulator(s) of distinct human T and B cell functions. J. Immunol. 127 427–429. [PubMed] [Google Scholar]
- Capon, C., Piller, F., Wieruszeski, J.M., Leroy, Y., and Fournet, B. 1990. Structural analysis of the carbohydrate chain isolated from Jacalin lectin. Carbohydr. Res. 121 1–1. [Google Scholar]
- Chipman, D.M., Grisaro, V., and Sharon, N. 1967. The binding of oligosaccharides containing N-acetylglucosamine and N-acetylmuramic acid to lysozyme. The specificity of binding subsites. J. Biol. Chem. 242 4388–4394. [PubMed] [Google Scholar]
- Corbeau, P., Haran, M., Binz, H., and Devaux, C. 1994. Jacalin, a lectin with anti-HIV-1 properties, and HIV-1 gp120 envelope protein interact with distinct regions of the CD4 molecule. Mol. Immunol. 31 569–575. [DOI] [PubMed] [Google Scholar]
- Dougherty, T.J., Kaufman, J.E., Goldfarb, A., Weishaupt, K.R., Boyle, D., and Mittleman, A. 1978. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 38 2628–2635. [PubMed] [Google Scholar]
- Eftink, M.R. and Ghiron, C.A. 1981. Fluorescence quenching studies with proteins. Anal. Biochem. 114199–227. [DOI] [PubMed] [Google Scholar]
- Favero, J., Corbeau, P., Nicolas, M., Benkirane, M., Trave, G., Dixon, J.F., Aucouturier, P., Rasheed, S., Parker, J.W., Liautard, J.P., et al. 1993. Inhibition of human immunodeficiency virus infection by the lectin jacalin and by a derived peptide showing a sequence similarity with gp120. Eur. J. Immunol. 23 179–185. [DOI] [PubMed] [Google Scholar]
- Gaikwad, S.M. and Khan, M.I. 2003. pH-dependent aggregation of oligomeric Artocarpus hirsuta lectin on thermal denaturation. Biochem. Biophys. Res. Commun. 311 254–257. [DOI] [PubMed] [Google Scholar]
- Gaikwad, S.M., Gurjar, M.M., and Khan, M.I. 1998. Fluorimetric studies on saccharide binding to the basic lectin from Artocarpus hirsuta. Biochem. Mol. Biol. Int. 46 1–9. [DOI] [PubMed] [Google Scholar]
- ——— 2002. Artocarpus hirsuta lectin. Differential modes of chemical and thermal denaturation. Eur. J. Biochem. 269 1413–1417. [DOI] [PubMed] [Google Scholar]
- Hirel, P.H., Schmitter, M.J., Dessen, P., Fayat, G., and Blanquet, S. 1989. Extent of N-terminal methionine excision from Escherichia coli proteins is governed by the side-chain length of the penultimate amino acid. Proc. Natl. Acad. Sci. 86 8247–8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houles, A.C., Peumans, W.J., van Damme, E.J., Barre, A., Bourne, Y., and Rouge, P. 2002. The size, shape and specificity of the sugar binding site of the jacalin-related lectins is profoundly affected by the proteolytic cleavage of the subunits. Biochem. J. 367 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaprakash, A.A., Geetha, R.P., Banuprakash, R.G., Banumathi, S., Betzel, C., Sekar, K., Surolia, A., and Vijayan, M. 2002. Crystal structure of the jacalin-T-antigen complex and a comparative study of lectin-T-antigen complexes. J. Mol. Biol. 321 637–645. [DOI] [PubMed] [Google Scholar]
- Jeyaprakash, A.A., Katiyar, S., Swaminathan, C.P., Sekar, K., Surolia, A., and Vijayan, M. 2003. Structural basis of the carbohydrate specificities of jacalin: An X-ray and modeling study. J. Mol. Biol. 332 217–228. [DOI] [PubMed] [Google Scholar]
- Kabir, S. 1998. Jacalin: A jackfruit (Artocarpus heterophyllus) seed-derived lectin of versatile applications in immunobiological research. J. Immunol. Methods 212 193–211. [DOI] [PubMed] [Google Scholar]
- Komath, S.S., Bhanu, K., Maiya, B.G., and Swamy, M.J. 2000. Binding of porphyrins by the tumor-specific lectin, jacalin [Jack fruit (Artocarpus integrifolia) agglutinin]. Biosci. Rep. 20 265–276. [DOI] [PubMed] [Google Scholar]
- Lafont, V., Dornand, J., d’Angeac, A.D., Monier, S., Alcover, A., and Favero, J. 1994. Jacalin, a lectin that inhibits in vitro HIV-1 infection, induces intracellular calcium increase via CD4 in cells lacking the CD3/TcR complex. J. Leukoc. Biol. 56 521–524. [DOI] [PubMed] [Google Scholar]
- Lafont, V., Dornand, J., Covassin, L., Liautard, J.P., and Favero, J. 1996. The lectin jacalin triggers CD4-mediated lymphocyte signaling by binding CD4 through a protein-protein interaction. J. Leukoc. Biol. 59 691–696. [DOI] [PubMed] [Google Scholar]
- Lathrop, B.K., Burack, W.R., Biltonen, R.L., and Rule, G.S. 1992. Expression of a group II phospholipase A2 from the venom of Agkistrodon piscivorus piscivorus in Escherichia coli: Recovery and renaturation from bacterial inclusion bodies. Protein Expr. Purif. 3 512–517. [DOI] [PubMed] [Google Scholar]
- Lehrer, S.S. and Leavis, P.C. 1978. Solute quenching of protein fluorescence. Methods Enzymol. 49 222–236. [DOI] [PubMed] [Google Scholar]
- Levy, J.G. 1995. Photodynamic therapy. Trends Biotechnol. 13 14–18. [DOI] [PubMed] [Google Scholar]
- Mahanta, S.K., Sastry, M.V., and Surolia, A. 1990. Topography of the combining region of a Thomsen-Friedenreich-antigen-specific lectin jacalin (Artocarpus integrifolia agglutinin). A thermodynamic and circular-dichroism spectroscopic study. Biochem. J. 265 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanta, S.K., Sanker, S., Rao, N.V., Swamy, M.J., and Surolia, A. 1992. Primary structure of a Thomsen-Friedenreich-antigen-specific lectin, jacalin [Artocarpus integrifolia (jack fruit) agglutinin]. Evidence for the presence of an internal repeat. Biochem. J. 284(Pt. 1): 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau, N., Brugier, J.C., Breux, J.P., Becq-Giraudon, B., Descamps, J.M., Aucouturier, P., and Preud’homme, J.L. 1989. Stimulation of peripheral blood lymphocytes of HIV-infected patients by jacalin, a lectin mitogenic for human CD4+ lymphocytes. AIDS 3 659–663. [DOI] [PubMed] [Google Scholar]
- Pineau, N., Aucouturier, P., Brugier, J.C., and Preud’homme, J.L. 1990. Jacalin: A lectin mitogenic for human CD4 T lymphocytes. Clin. Exp. Immunol. 80 420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, K.N., Suresh, C.G., Katre, U.V., Gaikwad, S.M., and Khan, M.I. 2004. Two orthorhombic crystal structures of a galactose-specific lectin from Artocarpus hirsuta in complex with methy-a-D-Galactose. Acta. Crystallogr. D. Biol. Crystallogr. 60 1404–1412. [DOI] [PubMed] [Google Scholar]
- Roque-Barreira, M.C. and Campos-Neto, A. 1985. Jacalin: An IgA-binding lectin. J. Immunol. 134 1740–1743. [PubMed] [Google Scholar]
- Ruffet, E., Paquet, N., Frutiger, S., Hughes, G.J., and Jaton, J.C. 1992. Structural and electron-microscopic studies of jacalin from jackfruit (Artocarpus integrifolia) show that this lectin is a 65 kDa tetramer. Biochem. J. 286 (Pt. 1): 131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry, M.V. and Surolia, A. 1986. Intrinsic fluorescence studies on saccharide binding to Artocarpus integrifolia lectin. Biosci. Rep. 6 853–860. [DOI] [PubMed] [Google Scholar]
- Sastry, M.V., Banarjee, P., Patanjali, S.R., Swamy, M.J., Swarnalatha, G.V., and Surolia, A. 1986. Analysis of saccharide binding to Artocarpus integrifolia lectin reveals specific recognition of T-antigen (β-D-Gal(1—3)D-GalNAc). J. Biol. Chem. 261 11726–11733. [PubMed] [Google Scholar]
- Semisotnov, G.V., Rodionova, N.A., Razgulyaev, O.I., Uversky, V.N., Gripas’, A.F., and Gilmanshin, R.I. 1991. Study of the “molten globule” intermediate state in protein folding by a hydrophobic fluorescent probe. Biopolymers 31 119–128. [DOI] [PubMed] [Google Scholar]
- Stryer, L. 1965. The interaction of a naphthalene dye with apomyoglobin and apohemoglobin. A fluorescent probe of non-polar binding sites. J. Mol. Biol. 13 482–495. [DOI] [PubMed] [Google Scholar]
- Tamma, S.M., Oyaizu, N., McCloskey, T.W., Kalyanaraman, V.S., and Pahwa, S. 1996. HIV-1 gp 120 blocks jacalin-induced proliferative response in CD4+ T cells: Jacalin as a useful surrogate marker for qualitative and quantitative deficiency of CD4+ T cells in HIV-1 infection. Clin. Immunol. Immunopathol. 80 290–297. [DOI] [PubMed] [Google Scholar]
- Tobias, J.W., Shrader, T.E., Rocap, G., and Varshavsky, A. 1991. The N-end rule in bacteria. Science 254 1374–1377. [DOI] [PubMed] [Google Scholar]
- Vandana, S., Raje, M., and Krishnasastry, M.V. 1997. The role of the amino terminus in the kinetics and assembly of α-hemolysin of Staphylococcus aureus. J. Biol. Chem. 272 24858–24863. [DOI] [PubMed] [Google Scholar]
- Yang, H. and Czapla, T.H. 1993. Isolation and characterization of cDNA clones encoding jacalin isolectins. J. Biol. Chem. 268 5905–5910. [PubMed] [Google Scholar]