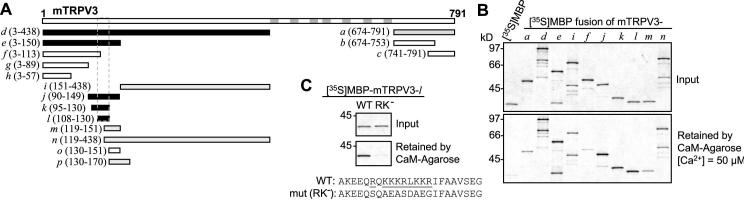
Figure 5. Identification of a CaM-binding site from the N-terminus of TRPV3.
A, diagram of murine TRPV3 and maltose-binding protein (MBP)-TRPV3 fusion proteins tested. Fragments of TRPV3 were prepared as MBP-fusion proteins and tested for binding to CaM in the presence of 50 μM Ca2+. The fusion proteins were designated as a, b, c, etc. and their positions in the full-length TRPV3 are indicated in parentheses. Black, gray, and open bars represent positive, weakly positive, and negative binding to CaM, respectively. The borders of the identified N-terminal CaM-binding site are shown by dashed lines. Gray bars in the full-length TRPV3 indicate transmembrane segments and the pore loop. B, representative binding results showing the sizes and the amounts of 35S-labeled MBP-TRPV3 fusion proteins added to the binding reactions (upper graph) and the amounts of the fusion proteins retained by CaM-agarose in the presence of 50 μM Ca2+ (lower graph). MBP was included alone as a negative control (lane 1). C, R113QKKKRLKKR122 was mutated to SQAEASDAEG as described in Materials and Methods. The mutated l fragment was fused to MBP and tested for binding to CaM under the same condition as in B. The graphs show that the mutant (RK-) does not bind to CaM.