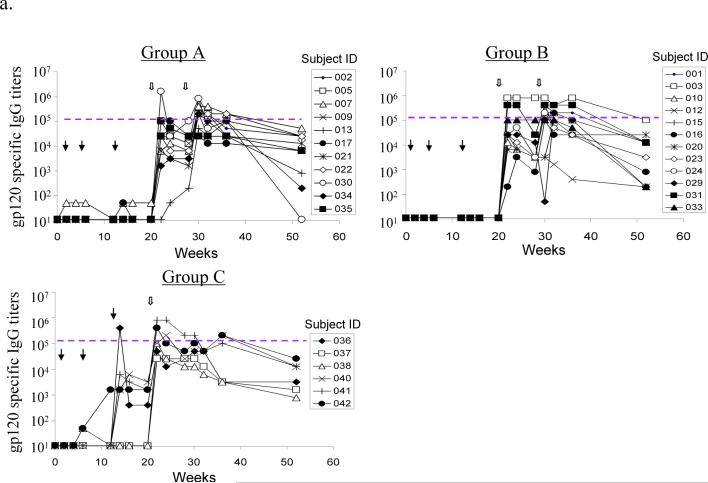
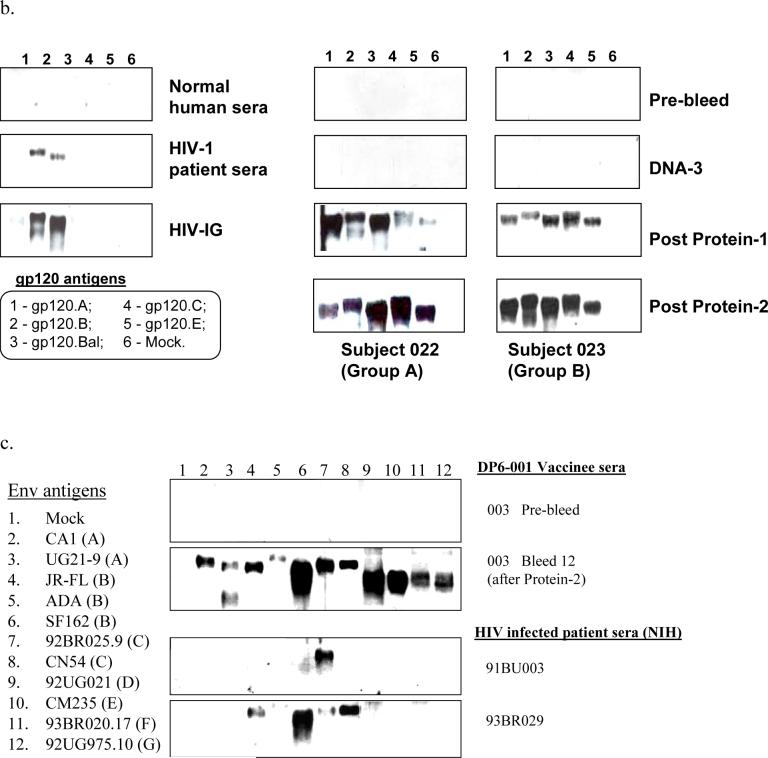
Figure 2.
DP6−001 formulation induced HIV-1 gp120-specific antibody responses in volunteers' sera. (a) Titers of serum gp120-specific IgG were measured by ELISA in individual volunteers of different study groups. Each curve represents one volunteer. The solid arrows indicate DNA immunizations and the open arrows indicate protein immunizations. The dotted line denotes the average titer of gp120-specific IgG in sera of three patients chronically infected with HIV-1 (titer range: 1:102,400 to 1:204,800). (b) Reactivity of two DP6−001 immune sera (#022 of Group A and #023 of Group B at 1:100 dilution) at different time points of immunization against five autologous primary HIV-1 gp120 glycoproteins as measured by Western blot analysis. Normal human sera and HIV-1 positive patient sera, both at 1:100 dilution, or HIV-1 positive immunoglobulin (HIV-IG) (at 0.5 mg/ml total human IgG) were included as controls. (c) Cross reactivity of sera from one representative DP6−001 immune serum #003 (Group B) against a panel of heterologous primary HIV-1 gp120 glycoproteins by Western blot analysis. Two individual HIV+ patient sera (91BU003 and 93BR029) were included as the controls. Serum dilution of 1:100 was used for both DP6−001 and HIV+ patient sera.